Abstract
Human mammaglobin is a member of the uteroglobin proteins family that has recently been tested as a specific marker for breast cancer. While low levels may be seen in normal breast tissue, expression is increased dramatically in breast cancer and is correlated with higher grade. Detection in blood and body fluids is also correlated with cancer metastasis, and its levels with prognosis. This promises to be a useful screen for early detection of breast cancer, especially in high risk individuals. Mammoglobin has also been used for immunotherapeutic targeting of breast cancer cells. However, there are some controversies regarding its diagnostic efficacy and prognostic value, which warrant further study.
Keywords: Breast cancer, diagnosis, immunotherapy, mammaglobin, prognosis
Introduction
Despite advances in the biology of cancer, there remains a growing need for improved diagnostic accuracy1,2. In most tumours, there is a lack of tissue or serum molecular markers with sufficient sensitivity and/or specificity for detection, and disease evolution. For breast cancer, carcinoembryonic antigen (CEA) and CA 15-3 (CA 27-29) are common markers, although these lack sensitivity and specificity, are rarely elevated prior to gross disease, and are not seen in many patients with metastases3,4. Many other biomarkers have been suggested for use in detecting breast cancer in tissues, peripheral blood and/or bone marrow including human mammaglobin, cytokeratins (CK19 & CK20), survivin, polymorphic epithelial mucin (MUC-1), epidermal growth factor receptor (EGFR), maspin, estrogen receptor (ER) and progesterone receptor (PR), urokinase plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1) and B726P and small breast epithelial mucin (SBEM)5,6,7,8,9. Most, however, are neither sufficiently sensitive, nor tumour-specific, to be clinically useful10. Clinically, ER, PR and human epidermal growth factor receptor 2 (HER-2) are the most useful markers for prognosis and therapy but not for diagnosis or evolution8,11,12. The search for new markers in breast cancer is still ongoing. This review attempts to evaluate human mammaglobin as a promising diagnostic, prognostic and potentially therapeutic tool in breast cancer.
Discovery and nomenclature of human mammaglobin
In 1994, Watson and Fleming recognized sequence tags expressed in neoplastic mammary epithelial tissue by using a modified differential display polymerase chain reaction (PCR) technique which was developed to detect and compare mRNA differential expression among cells13. One of these sequence tags led to the discovery and isolation of a novel full length cDNA that encoded a protein, now known as the human mammaglobin (hMAG)10. Human mammaglobins A and B are homologues and members of a large family, and subsequently have been repeatedly reported as potentially valuable in breast cancer diagnosis and prognosis14,15,16,17,18. Nevertheless, hMAG-B subtypes (hMAG B-1 and B-2) have also been detected in endometrioid endometrial carcinoma especially in well- and moderately-differentiated tumours19,20,21,22,23, and hMAG A was also detected in a case of a poorly differentiated myoepithelial cell rich carcinoma, though this may have been of breast origin24. The nomenclature used in the literature shows various other names and abbreviations for mammaglobin variants and their genes, such as MAM, MGB, UGB3, MMG, SCGB2A1. In this review, the abbreviations quoted will be restricted to hMAG-A and hMAG-B unless otherwise stated.
Chemistry and regulation of hMAG
Human MAG was described as one of the 23 members of the uteroglobin/Clara cell protein family of small epithelial secretory proteins, the secretoglobins25,26,27. It is a 93-amino acid protein with two N-linked glycosylation sites, both linked to approximately 3 kDa carbohydrate chains, with molecular masses of 23.4 and 16.2 kDa for the glycosylated and deglycosylated complexes, respectively27. Mammaglobin forms soluble, covalent heterodimers with lipophilin B in an anti-parallel manner that allows the formation of three disulphide bridges between the two molecules28,29. The mammaglobin protein forms four alpha helices that sandwich in a head-to-tail orientation creating a hydrophobic core, allowing the formation of the three closely situated disulfide bonds. In this model, an N-terminal cysteine of mammaglobin is covalently linked to the C-terminal cysteine of lipophilin B. The protein serological marker in breast cancer appears to reside in the sequences and framework of the hMAG-lipophilin complex28. The multigene family of MAG A and MAG B is localized on chromosome 11q12.2 in a dense cluster spanning not more than 400 kbp. In this same gene locus, the human Clara Cell 10-kDa protein (CC10) gene, mammaglobins A and B, lipophilins A and B, lacryglobin, and another member called lymphoglobin, are included26,30.
By Western blotting analysis, the MAG-A protein was found to exist in the mammary tissue in two main forms with approximate molecular masses of 18 and 25 kDa. Both forms were detected more frequently in breast carcinomas than in fibroadenomas or in normal breast tissues. Furthermore, an inverse relationship was found between the high molecular weight form of MAG and both tumour grade and proliferative index. No significant correlation was found between the MAG proteins and either tumour size or nodal status, although it was associated with a favourable prognosis for breast cancer31.
Although the secretoglobins are known to be regulated by steroid hormones, mammaglobin expression is not induced by estrogens in ER-positive breast cancer cell lines, such as MCF7 and T47D implying that mammaglobin is almost exclusively expressed in the mammary gland independent of steroid hormones32. Thus, PEA-3 expression vector and some repetitive sequences may be involved in the regulation of hMAG expression33. In breast tumours, hMAG A and lipophilin appear to be co-expressed simultaneously, suggesting the existence of common regulatory mechanisms16.
Function of mammaglobin protein
The function of mammaglobin protein is not known. Northern blot and RT-PCR analyses have demonstrated that hMAG expression is restricted to the mammary gland and that hMAG mRNA is highly detectable in human breast tumour cell lines and primary breast tumours compared to non malignant breast tissue27,32. However, its expression did not increase the growth rate of cell lines, indicating that it is not likely to be involved in a cell division function34. It was also expected to be readily secreted from breast tumours and to elicit production of autoantibodies detectable in the serum of breast cancer patients and women at high risk of breast cancer35 raising the question of whether this may be used as a marker. Moreover, the hydrophobic core is capable of binding steroid-like molecules, suggesting the existence of a hormonal transport or activation function28,36.
Mammaglobin in breast cancer
In preliminary attempts at determining the clinical utility of mammaglobin as a breast tumour marker, Watson and Fleming27 found that mammaglobin mRNA expression was multiplied by at least 10-fold when compared to normal breast tissue, using RT-PCR and Northern blot analysis, whereas by immunohistochemistry, they detected hMAG in 91 per cent of the breast cancer cases, independent of stage and histological type35. Similarly, a number of subsequent studies described the detection of mammaglobin at high levels in primary breast cancers, while it was either undetectable in non-breast tumours or present at low levels in healthy breast tissue, but not in other tissues, making it a suitable candidate for diagnosis of breast cancer37,38,39,40,41,42,43,44. Human MAG was also used in differentiating between the different sub-types of breast carcinomas, such as luminal A and B, HER-2, basal-like carcinoma (BLC), and unclassified triple-negative carcinoma (UTNC) by established surrogate immunohistochemical profiles45. Furthermore, MAG-A positive expression by IHC staining was found in approximately 90 per cent of invasive ductal carcinoma and in 80 per cent of intraductal carcinoma46. In addition, the over-expression of the hMAG gene was correlated with high grade breast cancer37,47. Hence, the usefulness of hMAG in diagnosis stems from its abundant detection in breast tumours, the low existence in tissues and tumours other than breast, and its efficiency in detecting residual disease and predicting recurrence6. However, controversies have been reported as the correlation between blood hMAG and grading has fluctuated31,48, and hMAG was detected, by tissue microarray, in 44 of the 544 non-breast tumours49.
MAG in metastatic lesions and in the blood and fluids of breast cancer patients
The detection of hMAG has been reported, both in the blood and stem cells of about 60 per cent of patients with breast cancer50,51. However, only 11 per cent detection was reported by the Suchi group52. Using the nested reverse transcriptase polymerase chain reaction (RT-PCR) assay, hMAG was detected by the Zach group in the blood of about quarter of 114 breast cancer patients, and in about half (35/81) of those with metastatic disease, but in none of healthy controls53. The same group investigated hMAG expression in cases of suspected breast cancer and found it positive in 43 per cent of 81 patients with metastatic breast cancer, but in less than 3 per cent in other test groups54. The general implication from these studies is that hMAG can be a reliable indicator or detector of metastasis. In cases of occult tumours of unknown origins, it was also found that detection of hMAG mRNA may aid in detecting metastatic breast tumours and in detecting breast cancer micrometastasis55,56,57,58,59,60,61.
The detection of hMAG-B was reported to be more evident during the later stages of cancer whereas maspin was detectable during earlier stages62. However, the detection of maspin can be influenced by a number of cytokines whereas hMAG is not63. hMAG detection by RT-PCR may be very useful in detecting occult cells in breast cancer patients64.
Hence, mammaglobin may be an important tool for detecting primary or metastatic breast cancer, monitoring lymph nodes during and after surgery, and predicting disease outcomes and recurrences65. hMAG elevation was associated with distant recurrences and decreased survival periods, and has been of high specificity for lymph nodal involvement66,67. Furthermore, it was also elevated in brain metastases in breast cancer patients68,69, used in the diagnosis and detection of metastases in breast cancer patients with pleural effusions or other metastases and recurrences70,71,72,73,74,75. In addition, it could also help differentiate between cutaneous apocrine carcinoma and breast metastases76, and in distinguishing metastatic breast carcinoma from a primary ovarian or uterine malignancy77 or abdominal cancers78. In detecting minimum residual disease in the bone marrow of breast cancer patients, MAG-A, along with maspin, was also useful6,59,79. The use of fluorescence in situ hybridization (FISH) along with RT-PCR, can improve the detection of metastases, contribute to the decision on tumour staging, assess treatment efficiency and indicate prognoses80.
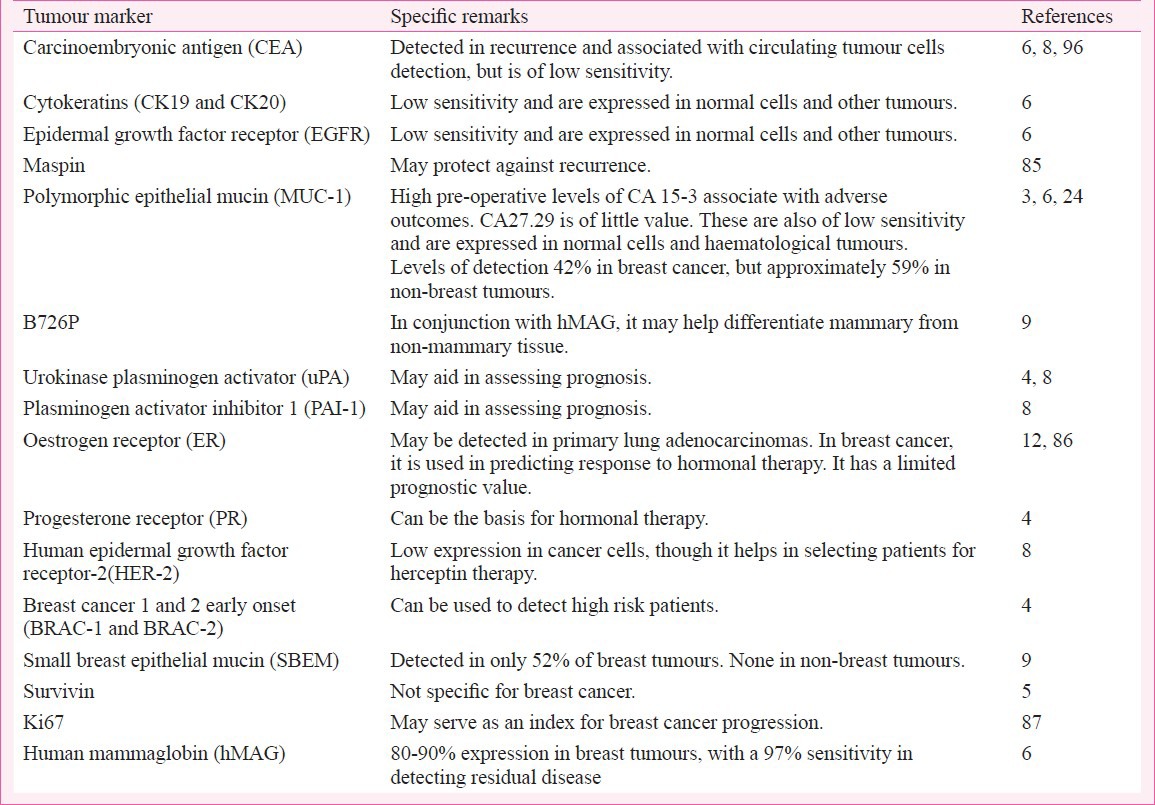
Altogether hMAG-A detection in breast cancer is of high specificity, but its sensitivity is low and can be enhanced by the simultaneous detection of Ki-6781. Generally, the expression of MAG-A along with B726P, small breast epithelial mucin (SBEM) and MUC1 was useful in differentiating breast tissues from non-breast tissues24. Although efficiency of the RT-PCR used can improve the detection82, critical views and findings have been reported with the implication that hMAG-A has only a limited value in identifying the mammary origin of metastatic carcinomas83. To further understand the significance of hMAG, a direct comparison of the clinical validity of hMAG with other markers, was done and summarized in the Table. hMAG mRNA appears to be more stable and detectable in the blood for relatively longer periods than other breast cancer markers, making it useful where samples are stored for short periods88.
Table.
A comparison of the efficiencies of hMAG with various other breast cancer markers as reported in the literature
Mammaglobin detection in lymph nodes
Although MAG and CEA were detected in all breast cancer cell lines tested, these were not detected in normal lymph nodes87. Similarly mammaglobin was detected in 100 per cent of histologically positive breast cancer lymph node samples, whereas no mammaglobin expression was observed in lymph nodes and in sentinel lymph nodes from cases without histologically detectable breast tumour cells40,64,66,89,90,91,92. In addition, the experimental set-up of conjugating the near infra red (NIR) fluoresecent dye VivoTag-S 680 to a murine monoclonal antibody against human mammaglobin-A was a promising additive as an imaging probe for the non-invasive detection of breast tumour metastasis in lymph nodes in mice46. The detection of circulating tumour cells (CTCs) was found to be enhanced by using a combination of markers along with hMAG such as cytokeratin 19, and this combination can be useful clinically for intra- or post-operative axillary lymph node dissection decisions in breast lymph nodes93,94,95, although cytokeratin 19 was found to be more specific than hMAG, when probed by RT-PCR in lymph nodal detection of breast cancer96. However, RT-PCR detection of hMAG was described as being more informative than both cytokeratin 19 and CEA regarding the molecular detection in sentinel lymph nodes97.
Mammaglobin correlation with other breast cancer markers
The analysis of over 300 tumours revealed that hMAG-A and lipophilin are co-expressed simultaneously16. Although mammaglobin is more specific and sensitive than epidermal growth factor receptor (EGF-R) and cytokeratin-1998, when tested with CEA and CA-15.3 simultaneously, its sensitivity was increased from 54 per cent to approximately 90 per cent, suggesting the usefulness of mammaglobin mRNA as an adjunct to these markers84. Samples from breast cancer patients, patients with benign breast tumours, healthy individuals, and patients with other solid tumours, investigated by quantitative RT-PCR for hMAG, survivin and human telomerase reverse transcriptase (hTERT) have shown that the three markers together, make up a powerful tool for detecting CTCs of breast cancer, with very high specificity, and that these were correlated with lymph nodal involvement. hMAG mRNA was specific for breast cancer, whereas survivin and hTERT were detected in the serum samples of patients with other solid tumours99. Other groups investigated parallel testing of the markers CEA mRNA, CK-19, HER-2, MAG-A, MAGEA3 (melanoma antigen family A, 3), TWIST homolog 1(Drosophila)] (TWIST-1) and hydroxymethylbilane synthase (HMBS). This appeared to increase the sensitivity for detecting CTCs with higher detection rates found in cases with metastasis100. Controversial results appeared regarding the correlation of hMAG expression with ER positive and negative tumours53,101,102. Correlations of hMAG with Ki67, ER status and other clinicopathological factors have been found to increase the detection and diagnostic efficiency, and it was also concluded that hMAG correlated, for unknown reasons, with the G3 histologic grading of breast cancer43,82. There were also associations between MAG expression and gross cystic disease fluid protein 15 (GCDFP-15) and with the molecular sub-types of breast carcinoma, such as basal-like carcinoma (BLC), and unclassified triple-negative carcinoma (UTNC). These same markers can be useful in detecting recurrences and distant metastases of breast tumours including detecting these in pleural effusions45,71,76,103. Similarly, a combination of markers including mammaglobin detected by immunohistochemistry (IHC) stains raised the sensitivity and specificity in distinguishing between cutaneous metastases of breast carcinomas (CMBCs) and sweat gland carcinomas (SGCs)104. In conclusion, in addition to the efficiency of hMAG in diagnosis and in detecting micrometastasis, its efficiency can be magnified by combining it with other markers.
Mammaglobin in assessing prognosis of breast cancer
Tumour size, extent of axillary node involvement, high histologic grade, bone marrow involvement, and the detection of CTCs are considered strong predictors of breast cancer recurrence, and of poor prognosis for patients with metastatic tumours105,106. Disseminated tumour cells in the bone marrow or in the blood, assessed by TWIST1, CK19 and MAG A mRNA, in addition to trefoil factor 1 (TFF-1) were correlated with short recurrence periods and poor outcomes67,107,108. Furthermore, hMAG detection in CTCs of the peripheral blood was a poor prognostic indicator47,102,109. Poor prognosis was also reported to be associated with detection of hMAG in leukapheresis products in individuals with high risk for breast cancer110. There are, however, controversies based on experimental findings in which either no correlation was established between hMAG detection in the blood of breast cancer patients and outcomes or other known prognostic factors111, or that mammaglobin was described as being a good prognostic indicator as well as being a useful indicator of metastases41, and the detection of hMAG mRNA in the blood of breast cancer patients did not correlate with the development of recurrences112. In addition, the expression of hMAG has been reported to be correlated with less aggressive mammary tumours113. Moreover, in vitro observations showed that over-expression of hMAG did not have a great effect on the growth rate of the breast carcinoma cell line Hs578T34. Hence, the views on the relationship between hMAG and prognosis are highly controversial.
The controversies reported may have been attributed to the ethnic differences as well as technical differences such as the blood volume used, in addition to the sensitivity of the assays used especially regarding the selection of primers and probes as well as the individual difference among patients. Moreover, immunohistochemical detection might have been accompanied by some bias in interpretation in different laboratories using kits and antibodies of different antigen detecting specificities and of dissimilar affinities.
Human MAG and cancer immunotherapy
In addition to its diagnostic role, hMAG also provided a new tool for the prognosis and management of breast cancer patients35,51. It was also concluded that the efficiency of therapy may be assessed by measuring the levels of hMAG mRNA by RT-PCR114. Also, a higher percentage of positive mammaglobin cases has been found among patients with metastatic and progressive breast cancer than in those responding to therapy115, as this may aid in detecting disseminated breast cancer and in making decisions for therapy69,116.
Human MAG may also enhance the possibility of establishing effective targeted therapies or vaccines against breast cancer cells through targeting mammaglobin-derived epitopes on cytotoxic T-lymphocytes117,118,119, for effective delivery of targeted therapies using a transmembrane N-terminal domain of hMAG120 or MAG-A-derived epitopes with HLA-A-2, HLA-A-3, and HLA-A-24, CD8 restricted responses121,122,123,124,125. Being a membrane-associated protein, hMAG may serve as a molecular marker for effective targeted drug therapies for breast cancer120. It may also aid in the preparation of experimental vaccines directed against breast cancer cells by introducing MAG-A DNA vaccine117, or by stimulating CD4(+)CD25(-) T cells in vitro with MAG-A-pulsed antigen-presenting cells124 or by transduction of dendritic cells with a Tat-MAG127. The notion that MAG can bind breast cancer cells has brought up speculations about its possible usefulness in directing radioisotopes or toxins to target these cells128. Another suggested therapeutic approach has been the use of the MAG promoter to deliver, by gene therapy, oncolytic viruses or toxic genes to mammary tumours129. Moreover, a combination of targets such as hMAG combined with HER-2/neu in active immunotherapy may enhance the therapeutic vaccine efficiency and specific T cells for use in adoptive immunotherapy for the treatment of established metastatic disease130. Nevertheless, a recent report stated that upregulation to induce over-expression of h-MAG in breast cancer cells can reduce the metastatic potential of these cells131. This approach may prove to have an additive value in the management of aggressive breast cancer.
Mammaglobin is a promising breast marker
Mammaglobin, known for its mammary tissue specificity, has been considered a promising diagnostic marker in breast cancer for almost 10 years. In particular, the application of mammaglobin RT-PCR to detect disseminated breast cancer cells has been reported. Much work has evaluated the detection of mammaglobin mRNA in lymph nodes, blood, and bone marrow of breast cancer patients. Structural details about the mammaglobin complex have also been discovered, and these findings can be implemented to optimize detection of the secreted protein18. The peculiarity of hMAG lies in its almost sole existence in mammary tissue and mammary carcinoma. In addition, the heightened expression in carcinomas and its association with tumour grades renders it an excellent marker for diagnosis and prognosis. Methods are being studied to screen and detect early breast cancer. To date, only mammography has been the method of early detection and hMAG may enhance and complement the predictive value of mammography9.
Conclusions
Looking at the various reports and opinions laid down in the literature, human MAG does carry the potential of being a molecule of diagnostic, prognostic, as well as therapeutic significance. Furthermore, it appears to have an additional potential usefulness as a screening tool for breast cancer. More studies will have to be analysed to explain the differences of results found by some groups.
Acknowledgment
The author acknowledges the Royal College of Medicine Perak, University Kuala Lumpur, Malaysia, for encouraging this work to be accomplished. Thanks are also due to Prof Dr Jason F. Cabot, Royal College of Medicine Perak, for his critical comments on this manuscript.
References
- 1.Smith JA, Andreopoulou E. An overview of the status of imaging screening technology for breast cancer. Ann Oncol. 2004;15(Suppl 1):I18–26. doi: 10.1093/annonc/mdh653. [DOI] [PubMed] [Google Scholar]
- 2.Szoke J, Udvarhelyi N. Modern pathologic diagnostics in breast cancer. Orv Hetil. 2012;153:22–30. doi: 10.1556/OH.2012.29251. [DOI] [PubMed] [Google Scholar]
- 3.Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–51. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 4.Marić P, Ozretić P, Levanat S, Oresković S, Antunac K, Beketiæ-Oreskoviæ L. Tumor markers in breast cancer-evaluation of their clinical usefulness. Coll Antropol. 2011;35:241–7. [PubMed] [Google Scholar]
- 5.Al-Joudi FS, Iskandar ZA, Hasnan J, Rusli J, Kamal Y, Imran AK, et al. Expression of survivin and its clinicopathological correlations in invasive ductal carcinoma of the breast. Singapore Med J. 2007;48:607–14. [PubMed] [Google Scholar]
- 6.Corradini P, Voena C, Astolfi M, Delloro S, Pilotti S, Arrigoni G, et al. Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol. 2001;12:1693–8. doi: 10.1023/a:1013573108945. [DOI] [PubMed] [Google Scholar]
- 7.Jarvinen TA, Pelto-Huikko M, Holli K, Isola J. Oestrogen receptor beta is coexpressed with ERalpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, et al. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–93. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien N, O’Donovan N, Foley D, Hill AD, McDermott E, O’Higgins N, et al. Use of a panel of novel genes for differentiating breast cancer from non-breast tissues. Tumour Biol. 2007;28:312–7. doi: 10.1159/000115527. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein JL, Godbold JH, Raptis G, Watson MA, Levinson B, Aaronson SA, et al. Identification of mammaglobin as a novel serum marker for breast cancer. Clin Cancer Res. 2005;11:6528–35. doi: 10.1158/1078-0432.CCR-05-0415. [DOI] [PubMed] [Google Scholar]
- 11.Leong AS, Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78:99–114. doi: 10.1159/000292644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen EV, Jordan VC. The oestrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–9. [PubMed] [Google Scholar]
- 13.Watson MA, Fleming TP. Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res. 1994;54:4598–602. [PubMed] [Google Scholar]
- 14.El-Sharkawy SL, El-Aal WE, El-Shaer MA, Abbas NF, Youssef MF. Mammaglobin: a novel tumor marker for breast cancer. Turkish J Cancer. 2007:89–97. [Google Scholar]
- 15.O’Brien N, Maguire TM, O’Donovan N, Lynch N, Hill AD, McDermott E, et al. Mammaglobin a: a promising marker for breast cancer. Clin Chem. 2002;48:1362–4. [PubMed] [Google Scholar]
- 16.Span PN, Waanders E, Manders P, Heuvel JJ, Foekens JA, Watson MA, et al. Mammaglobin is associated with low-grade, steroid receptor-positive breast tumors from postmenopausal patients, and has independent prognostic value for relapse-free survival time. J Clin Oncol. 2004;22:691–8. doi: 10.1200/JCO.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Zafrakas M, Petschke B, Donner A, Fritzsche F, Kristiansen G, Knüchel R, et al. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer. 2006;6:88. doi: 10.1186/1471-2407-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zehentner BK, Carter D. Mammaglobin: a candidate diagnostic marker for breast cancer. Clin Biochem. 2004:249–57. doi: 10.1016/j.clinbiochem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Grünewald K, Haun M, Fiegl M, Urbanek M, Müller-Holzner E, Massoner A, et al. Mammaglobin expression in gynecologic malignancies and malignant effusions detected by nested reverse transcriptase-polymerase chain reaction. Lab Invest. 2002;82:1147–53. doi: 10.1097/01.lab.0000027840.16064.8a. [DOI] [PubMed] [Google Scholar]
- 20.Onuma K, Dabbs DJ, Bhargava R. Mammaglobin expression in the female genital tract: immunohistochemical analysis in benign and neoplastic endocervix and endometrium. Int J Gynecol Pathol. 2008;27:418–25. doi: 10.1097/PGP.0b013e31815d05ec. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki E, Tsunoda N, Hatanaka Y, Mori N, Iwata H, Yatabe Y. Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol. 2007;20:208–14. doi: 10.1038/modpathol.3800731. [DOI] [PubMed] [Google Scholar]
- 22.Tassi RA, Bignotti E, Rossi E, Falchetti M, Donzelli C, Calza S, et al. Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol Oncol. 2007;105:578–85. doi: 10.1016/j.ygyno.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Tassi RA, Bignotti E, Falchetti M, Calza S, Ravaggi A, Rossi E, et al. Mammaglobin B expression in human endometrial cancer. Int J Gynecol Cancer. 2008;18:1090–6. doi: 10.1111/j.1525-1438.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonadio AG, Ferro P, Moroni M, Gorji N, Giannico A, Dessanti P, et al. Poorly differentiated breast carcinoma with an abundant myoepithelial component: morphologic and immunohistochemical features and mammaglobin gene expression. Pathologica. 2003;95:209–13. [PubMed] [Google Scholar]
- 25.Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, et al. Uteroglobin/Clara cell 10-kDa family of proteins : nomenclature committee report. Ann NY Acad Sci. 2000;923:348–54. doi: 10.1111/j.1749-6632.2000.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 26.Ni J, Kalff-Suske M, Gentz R, Schageman J, Beato M, Klug J. All human genes of the uteroglobin family are localized on chromosome 11q12.2 and form a dense cluster. Ann N Y Acad Sci. 2000;923:25–42. doi: 10.1111/j.1749-6632.2000.tb05517.x. [DOI] [PubMed] [Google Scholar]
- 27.Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–5. [PubMed] [Google Scholar]
- 28.Carter D, Douglass JF, Cornellison CD, Retter MW, Johnson JC, Bennington AA, et al. Purification and characterization of the mammaglobin/lipophilin B complex, a promising diagnostic marker for breast cancer. Biochemistry. 2002;41:6714–22. doi: 10.1021/bi0159884. [DOI] [PubMed] [Google Scholar]
- 29.Colpitts TL, Billing-Medel P, Friedman P, Granados EN, Hayden M, Hodges S, et al. Mammaglobin is found in breast tissue as a complex with BU101. Biochemistry. 2001;40:11048–59. doi: 10.1021/bi010284f. [DOI] [PubMed] [Google Scholar]
- 30.Becker RM, Darrow C, Zimonjic DB, Popescu NC, Watson MA, Fleming TP. Identification of mammaglobin B, a novel member of the uteroglobin gene family. Genomics. 1998;54:70–8. doi: 10.1006/geno.1998.5539. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien NA, O’Donovan N, Ryan B, Hill AD, McDermott E, O’Higgins N, et al. Mammaglobin a in breast cancer: existence of multiple molecular forms. Int J Cancer. 2005;114:623–7. doi: 10.1002/ijc.20780. [DOI] [PubMed] [Google Scholar]
- 32.Watson MA, Darrow C, Zimonjic DB, Popescu NC, Fleming TP. Structure and transcriptional regulation of the human mammaglobin gene, a breast cancer associated member of the uteroglobin gene family localized to chromosome11q13. Oncogene. 1998;16:817–24. doi: 10.1038/sj.onc.1201597. [DOI] [PubMed] [Google Scholar]
- 33.Hesselbrock DR, Kurpios N, Hassell JA, Watson MA, Fleming TP. PEA3, AP-1, and a unique repetitive sequence all are involved in transcriptional regulation of the breast cancer-associated gene, mammaglobin. Breast Cancer Res Treat. 2005;89:289–96. doi: 10.1007/s10549-004-2622-z. [DOI] [PubMed] [Google Scholar]
- 34.Sjödin A, Ljuslinder I, Henriksson R, Hedman H. Mammaglobin and lipophilin B expression in breast tumors and their lack of effect on breast cancer cell proliferation. Anticancer Res. 2008;28:1493–8. [PubMed] [Google Scholar]
- 35.Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59:3028–31. [PubMed] [Google Scholar]
- 36.Pattabiraman N, Matthews JH, Ward KB, Mantile-Selvaggi G, Miele L, Mukherjee AB. Crystal structure analysis of recombinant human uteroglobin and molecular modeling of ligand binding. Ann N Y Acad Sci. 2000;923:113–27. doi: 10.1111/j.1749-6632.2000.tb05523.x. [DOI] [PubMed] [Google Scholar]
- 37.Al-Joudi FS, Kaid FA, Ishak I, Mohamed N, Osman K, Alias IZ. Expression of human mammaglobin and clinicopathologic correlations in breast cancer: findings in Malaysia. Indian J Pathol Microbiol. 2011;54:284–9. doi: 10.4103/0377-4929.81596. [DOI] [PubMed] [Google Scholar]
- 38.Fritzsche FR, Thomas A, Winzer KJ, Beyer B, Dankof A, Bellach J, et al. Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer. Histol Histopathol. 2007;22:1221–30. doi: 10.14670/HH-22.1221. [DOI] [PubMed] [Google Scholar]
- 39.Houghton RL, Dillon DC, Molesh DA, Zehentner BK, Xu J, Jiang J, et al. Transcriptional complementarity in breast cancer: application to detection of circulating tumor cells. Mol Diagn. 2001;6:79–91. doi: 10.1007/BF03262038. [DOI] [PubMed] [Google Scholar]
- 40.Leygue E, Snell L, Dotzlaw H, Hole K, Troup S, Hiller-Hitchcock T, et al. Mammaglobin, a potential marker of breast cancer nodal metastasis. J Pathol. 1999;189:28–33. doi: 10.1002/(SICI)1096-9896(199909)189:1<28::AID-PATH389>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Raica M, Cîmpean AM, Meche A, Alexa A, Suciu C, Mureºan A. Analysis of the immunohistochemical expression of mammaglobin A in primary breast carcinoma and lymph node metastasis. Rom J Morphol Embryol. 2009;50:341–7. [PubMed] [Google Scholar]
- 42.Rehman F, Nagi AH, Hussain M. Immunohistochemical expression and correlation of mammaglobin with the grading system of breast carcinoma. Indian J Pathol Microbiol. 2010;53:619–23. doi: 10.4103/0377-4929.72000. [DOI] [PubMed] [Google Scholar]
- 43.Roncella S, Ferro P, Bacigalupo B, Dessanti P, Giannico A, Gorji N, et al. Relationship between human mammaglobin mRNA expression in breast cancer tissue and clinico-pathologic features of the tumors. J Exp Clin Cancer Res. 2006;25:65–72. [PubMed] [Google Scholar]
- 44.Silva AL, Tomé MJ, Correia AE, Passos-Coelho JL. Human mammaglobin RT-PCR assay for detection of occult breast cancer cells in hematopoietic products. Ann Oncol. 2002;13:422–9. doi: 10.1093/annonc/mdf107. [DOI] [PubMed] [Google Scholar]
- 45.Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587–91. doi: 10.1309/AJCPMFR6OA8ICHNH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung K. Bethesda (MD): National Center for Biotechnology Information (US); 2004. VivoTag-S 680-anti-human mammaglobin-A monoclonal antibody. Molecular Imaging and Contrast Agent Database (MICAD) [Internet] p. 13. [PubMed] [Google Scholar]
- 47.Mikhitarian K, Martin RH, Ruppel MB, Gillanders WE, Hoda R, Schutte del H, et al. Detection of mammaglobin mRNA in peripheral blood is associated with high grade breast cancer: interim results of a prospective cohort study. BMC Cancer. 2008;8:55. doi: 10.1186/1471-2407-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Attar NI, Gaefar HA. Plasma mammaglobin messenger RNA in breast cancer patients as an addition to serum tumor. Egypt J Immunol. 2007;14:111–21. [PubMed] [Google Scholar]
- 49.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–13. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 50.Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci. 2000;923:78–89. doi: 10.1111/j.1749-6632.2000.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 51.Fanger GR, Houghton RL, Retter MW, Hendrickson RC, Babcook J, Dillon DC, et al. Detection of mammaglobin in the sera of patients with breast cancer. Tumour Biol. 2002;23:212–21. doi: 10.1159/000067251. [DOI] [PubMed] [Google Scholar]
- 52.Suchy B, Austrup F, Driesel G, Eder C, Kusiak I, Uciechowski P, et al. Detection of mammaglobin expressing cells in blood of breast cancer patients. Cancer Lett. 2000;158:171–8. doi: 10.1016/s0304-3835(00)00520-6. [DOI] [PubMed] [Google Scholar]
- 53.Zach O, Kasparu H, Krieger O, Hehenwarter W, Girschikofsky M, Lutz D. Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via a nested reverse transcriptase polymerase chain reaction assay for mammaglobin mRNA. J Clin Oncol. 1999;17:2015–9. doi: 10.1200/JCO.1999.17.7.2015. [DOI] [PubMed] [Google Scholar]
- 54.Zach O, Kasparu H, Wagner H, Krieger O, Lutz D. Mammaglobin as a marker for the detection of tumor cells in the peripheral blood of breast cancer patients. Ann N Y Acad Sci. 2000;923:343–5. doi: 10.1111/j.1749-6632.2000.tb05547.x. [DOI] [PubMed] [Google Scholar]
- 55.Li G, Zhang J, Jin K, He K, Wang H, Lu H, et al. Human mammaglobin: a superior marker for reverse-transcriptase PCR in detecting circulating tumor cells in breast cancer patients. Biomark Med. 2011;5:249–60. doi: 10.2217/bmm.11.20. [DOI] [PubMed] [Google Scholar]
- 56.Han JH, Kang Y, Shin HC, Kim HS, Kang YM, Kim YB, et al. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330–4. doi: 10.5858/2003-127-1330-MEILNI. [DOI] [PubMed] [Google Scholar]
- 57.Aihara T, Fujiwara Y, Ooka M, Sakita I, Tamaki Y, Monden M. Mammaglobin B as a novel marker for detection of breast cancer micrometastases in axillary lymph nodes by reverse transcription-polymerase chain reaction. Breast Cancer Res Treat. 1999;58:137–40. doi: 10.1023/a:1006335817889. [DOI] [PubMed] [Google Scholar]
- 58.Ghaffari SR, Sabokbar T, Tahmasebi S, Dastan J, Shorakae S, Moradi A, et al. Combining mammaglobin and carcinoembryonic mRNA markers for early detection of micrometastases from breast cancers--a molecular study of 59 patients. Asian Pac J Cancer Prev. 2006;7:396–8. [PubMed] [Google Scholar]
- 59.Janku F, Kleibl Z, Novotny J, Tesarova P, Petruzelka L, Matous B. Mammaglobin A, a novel marker of minimal residual disease in early stages breast cancer. Neoplasma. 2004;51:204–8. [PubMed] [Google Scholar]
- 60.Liu N, Zhang W, Shao Y. Mammaglobin mRNA measurement in the detection of micrometastasis in peripheral blood of breast cancer patients. Zhonghua Zhong Liu Za Zhi. 2001;23:317–9. [PubMed] [Google Scholar]
- 61.Ntoulia M, Stathopoulou A, Ignatiadis M, Malamos N, Mavroudis D, Georgoulias V, et al. Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR. Clin Biochem. 2006;39:879–87. doi: 10.1016/j.clinbiochem.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Mercatali L, Valenti V, Calistri D, Calpona S, Rosti G, Folli S, et al. RT-PCR determination of maspin and mammaglobin B in peripheral blood of healthy donors and breast cancer patients. Ann Oncol. 2006;17:424–8. doi: 10.1093/annonc/mdj109. [DOI] [PubMed] [Google Scholar]
- 63.Ballestrero A, Garuti A, Bertolotto M, Rocco I, Boy D, Nencioni A, et al. Effect of different cytokines on mammaglobin and maspin gene expression in normal leukocytes: possible relevance to the assays for the detection of micrometastatic breast cancer. Br J Cancer. 2005;92:1948–52. doi: 10.1038/sj.bjc.6602563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roncella S, Ferro P, Bacigalupo B, Pronzato P, Tognoni A, Falco E, et al. Human mammaglobin mRNA is a reliable molecular marker for detecting occult breast cancer cells in peripheral blood. J Exp Clin Cancer Res. 2005;24:265–71. [PubMed] [Google Scholar]
- 65.Zehentner BK, Dillon DC, Jiang Y, Xu J, Bennington A, Molesh DA, et al. Application of a multigene reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem. 2002;48:1225–31. [PMC free article] [PubMed] [Google Scholar]
- 66.Ferro P, Franceschini MC, Bacigalupo B, Dessanti P, Falco E, Fontana V, et al. Detection of circulating tumour cells in breast cancer patients using human mammaglobin RT-PCR: association with clinical prognostic factors. Anticancer Res. 2010;30:2377–82. [PubMed] [Google Scholar]
- 67.Verbanac KM, Min CJ, Mannie AE, Lu J, O’Brien KF, Rosman M, et al. ELU/AAMC Sentinel Node Study Group. Long-term follow-up study of a prospective multicenter sentinel node trial: molecular detection of breast cancer sentinel node metastases. Ann Surg Oncol. 2010;17(Suppl 3):368–77. doi: 10.1245/s10434-010-1262-2. [DOI] [PubMed] [Google Scholar]
- 68.Dono M, Ferro P, Benedetti L, Capellini C, Moroni M, Dessanti P, et al. Molecular detection of human mammaglobin in cerebrospinal fluid from breast cancer patient with leptomeningeal carcinomatosis. J Neurooncol. 2009;91:295–8. doi: 10.1007/s11060-008-9711-5. [DOI] [PubMed] [Google Scholar]
- 69.Dono M, Ferro P, Franceschini MC, Dessanti P, Bacigalupo B, Cibei E, et al. Human mammaglobin transcript amplification for differential diagnosis in a breast cancer metastatic to dura mater. Anticancer Res. 2011;31:1061–4. [PubMed] [Google Scholar]
- 70.Chia SY, Thike AA, Cheok PY, Tan PH. Utility of mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15) in confirming a breast origin for recurrent tumors. Breast. 2010;19:355–9. doi: 10.1016/j.breast.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Ciampa A, Fanger G, Khan A, Rock KL, Xu B. Mammaglobin and CRxA-01 in pleural effusion cytology: potential utility of distinguishing metastatic breast carcinomas from other cytokeratin 7-positive/cytokeratin 20-negative carcinomas. Cancer. 2004;102:368–72. doi: 10.1002/cncr.20627. [DOI] [PubMed] [Google Scholar]
- 72.Roncella S, Ferro P, Bacigalupo B, Dessanti P, Pronzato P, Franceschini MC, et al. Assessment of RT-PCR detection of human mammaglobin for the diagnosis of breast cancer derived pleural effusions. Diagn Mol Pathol. 2008;17:28–33. doi: 10.1097/PDM.0b013e31811ffe3b. [DOI] [PubMed] [Google Scholar]
- 73.Roncella S, Ferro P, Franceschini MC, Bacigalupo B, Dessanti P, Sivori M, et al. Diagnosis and origin determination of malignant pleural effusions through the use of the breast cancer marker human mammaglobin. Diagn Mol Pathol. 2010;19:92–8. doi: 10.1097/PDM.0b013e3181ba6c78. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Spaulding B, Sienko A, Liang Y, Li H, Nielsen G, et al. Mammaglobin, a valuable diagnostic marker for metastatic breast carcinoma. Int J Clin Exp Pathol. 2009;2:384–9. [PMC free article] [PubMed] [Google Scholar]
- 75.Yan Z, Gidley J, Horton D, Roberson J, Eltoum IE, Chhieng DC. Diagnostic utility of mammaglobin and GCDFP-15 in the identification of metastatic breast carcinoma in fluid specimens. Diagn Cytopathol. 2009;37:475–8. doi: 10.1002/dc.21039. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Flores A. Mammaglobin immunostaining in the differential diagnosis between cutaneous apocrine carcinoma and cutaneous metastasis from breast carcinoma. Cesk Patol. 2009;45:108–12. [PubMed] [Google Scholar]
- 77.Kanner WA, Galgano MT, Stoler MH, Mills SE, Atkins KA. Distinguishing breast carcinoma from Müllerian serous carcinoma with mammaglobin and mesothelin. Int J Gynecol Pathol. 2008;27:491–5. doi: 10.1097/PGP.0b013e31817d5340. [DOI] [PubMed] [Google Scholar]
- 78.Aihara T, Fujiwara Y, Miyake Y, Okami J, Okada Y, Iwao K, et al. Mammaglobin B gene as a novel marker for lymph node micrometastasis in patients with abdominal cancers. Cancer Lett. 2000;150:79–84. doi: 10.1016/s0304-3835(99)00378-x. [DOI] [PubMed] [Google Scholar]
- 79.Bregni M, Fleischhauer K, Bernardi M, Pescarollo A, Guggiari E, Lunghi F, et al. Bone marrow mammaglobin expression as a marker of graft-versus-tumor effect after reduced-intensity allografting for advanced breast cancer. Bone Marrow Transplant. 2006;37:311–5. doi: 10.1038/sj.bmt.1705248. [DOI] [PubMed] [Google Scholar]
- 80.Fiegl M, Haun M, Massoner A, Krugmann J, Müller-Holzner E, Hack R, et al. Combination of cytology, fluorescence in situ hybridization for aneuploidy, and reverse-transcriptase polymerase chain reaction for human mammaglobin/ mammaglobin B expression improves diagnosis of malignant effusions. J Clin Oncol. 2004;22:474–83. doi: 10.1200/JCO.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 81.Gargano G, Agnese V, Calò V, Corsale S, Augello C, Bruno L, et al. Detection and quantification of mammaglobin in the blood of breast cancer patients: can it be useful as a potential clinical marker? Preliminary results of a GOIM (Gruppo Oncologico dell’Italia Meridionale) prospective study. Ann Oncol. 2006;17(Suppl 7):vii41–5. doi: 10.1093/annonc/mdl948. [DOI] [PubMed] [Google Scholar]
- 82.Cerveira N, Torres L, Rocha P, Bizarro S, Pereira D, Abreu J, et al. Highly sensitive detection of the MGB1 transcript (mammaglobin) in the peripheral blood of breast cancer patients. Int J Cancer. 2004;108:592–5. doi: 10.1002/ijc.11609. [DOI] [PubMed] [Google Scholar]
- 83.Reyes C, Gomez-Fernández C, Nadji M. Metaplastic and medullary mammary carcinomas do not express mammaglobin. Am J Clin Pathol. 2012;137:747–52. doi: 10.1309/AJCP5W5SEZSEHUHE. [DOI] [PubMed] [Google Scholar]
- 84.Lin YC, Wu Chou YH, Liao IC, Cheng AJ. The expression of mammaglobin mRNA in peripheral blood of metastatic breast cancer patients as an adjunct to serum tumor markers. Cancer Lett. 2003;191:93–9. doi: 10.1016/s0304-3835(02)00545-1. [DOI] [PubMed] [Google Scholar]
- 85.Ferrucci PF, Rabascio C, Gigli F, Corsini C, Giordano G, Bertolini F, Martinelli G. A new comprehensive gene expression panel to study tumor micrometastasis in patients with high-risk breast cancer. Int J Oncol. 2007;30:955–62. doi: 10.3892/ijo.30.4.955. [DOI] [PubMed] [Google Scholar]
- 86.Duffy MJ. Estrogen receptors: role in breast cancer. Crit Rev Clin Lab Sci. 2006;43:325–47. doi: 10.1080/10408360600739218. [DOI] [PubMed] [Google Scholar]
- 87.Min CJ, Tafra L, Verbanac KM. Identification of superior markers for polymerase chain reaction detection of breast cancer metastases in sentinel lymph nodes. Cancer Res. 1998;58:4581–4. [PubMed] [Google Scholar]
- 88.Benoy IH, Elst H, Van Dam P, Scharpé S, Van Marck E, Vermeulen PB, et al. Detection of circulating tumour cells in blood by quantitative real-time RT-PCR: effect of pre-analytical time. Clin Chem Lab Med. 2006;44:1082–7. doi: 10.1515/CCLM.2006.210. [DOI] [PubMed] [Google Scholar]
- 89.Dell’Orto P, Biasi MO, Del Curto B, Zurrida S, Galimberti V, Viale G. Assessing the status of axillary sentinel lymph nodes of breast carcinoma patients by a real-time quantitative RT-PCR assay for mammaglobin 1 mRNA. Breast Cancer Res Treat. 2006;98:185–90. doi: 10.1007/s10549-005-9148-x. [DOI] [PubMed] [Google Scholar]
- 90.Kataoka A, Mori M, Sadanaga N, Ueo H, Tsuji K, Rai Y, et al. RT-PCR detection of breast cancer cells in sentinel lymph nodes. Int J Oncol. 2000;16:1147–52. doi: 10.3892/ijo.16.6.1147. [DOI] [PubMed] [Google Scholar]
- 91.Marchetti A, Buttitta F, Bertacca G, Zavaglia K, Bevilacqua G, Angelucci D, et al. mRNA markers of breast cancer nodal metastases: comparison between mammaglobin and carcinoembryonic antigen in 248 patients. J Pathol. 2001;195:186–90. doi: 10.1002/path.943. [DOI] [PubMed] [Google Scholar]
- 92.Ouellette RJ, Richard D, Maïcas E. RT-PCR for mammaglobin genes, MGB1 and MGB2, identifies breast cancer micrometastases in sentinel lymph nodes. Am J Clin Pathol. 2004;121:637–43. doi: 10.1309/MMAC-TXT5-5L8Q-TKC1. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Zou TN, Wu ZP, Zhou YC, Gu YL, Liu X, et al. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer. Int J Biol Markers. 2010;25:59–68. doi: 10.1177/172460081002500201. [DOI] [PubMed] [Google Scholar]
- 94.Julian TB, Blumencranz P, Deck K, Whitworth P, Berry DA, Berry SM, et al. Novel intraoperative molecular test for sentinel lymph node metastases in patients with early-stage breast cancer. J Clin Oncol. 2008;26:3338–45. doi: 10.1200/JCO.2007.14.0665. [DOI] [PubMed] [Google Scholar]
- 95.Tjensvoll K, Oltedal S, Farmen RK, Shammas FV, Heikkilä R, Kvaløy JT, et al. Disseminated tumor cells in bone marrow assessed by TWIST1, cytokeratin 19, and mammaglobin A mRNA predict clinical outcome in operable breast cancer patients. Clin Breast Cancer. 2010;10:378–84. doi: 10.3816/CBC.2010.n.050. [DOI] [PubMed] [Google Scholar]
- 96.Berger J, Mueller-Holzner E, Fiegl H, Marth C, Daxenbichler G. Evaluation of three mRNA markers for the detection of lymph node metastases. Anticancer Res. 2006;26:3855–60. [PubMed] [Google Scholar]
- 97.Gimbergues P, Dauplat MM, Cayre A, Durando X, Le Bouedec G, Finat-Duclos F, et al. Correlation between molecular metastases in sentinel lymph nodes of breast cancer patients and St Gallen risk category. Eur J Surg Oncol. 2007;33:16–22. doi: 10.1016/j.ejso.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 98.Grünewald K, Haun M, Urbanek M, Fiegl M, Müller-Holzner E, Gunsilius E, et al. Mammaglobin gene expression: a superior marker of breast cancer cells in peripheral blood in comparison to epidermal-growth-factor receptor and cytokeratin-19. Lab Invest. 2000;80:1071–7. doi: 10.1038/labinvest.3780112. [DOI] [PubMed] [Google Scholar]
- 99.Shen C, Hu L, Xia L, Li Y. The detection of circulating tumor cells of breast cancer patients by using multimarker (Survivin, hTERT and hMAM) quantitative real-time PCR. Clin Biochem. 2009;42:194–200. doi: 10.1016/j.clinbiochem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem. 2011;57:421–30. doi: 10.1373/clinchem.2010.154328. [DOI] [PubMed] [Google Scholar]
- 101.Guan XF, Hamedani MK, Adeyinka A, Walker C, Kemp A, Murphy LC, et al. Relationship between mammaglobin expression and estrogen receptor status in breast tumors. Endocrine. 2003;21:245–50. doi: 10.1385/ENDO:21:3:245. [DOI] [PubMed] [Google Scholar]
- 102.Ceballos MP, Zumoffen C, Massa E, Cipulli G, Funes CC, Gil AB, et al. Detection of mammaglogin A in blood from breast cancer patients, before and after treatment, using a one-tube nested PCR protocol. Association with the absence of tumor estrogen receptors. Clin Biochem. 2011;44:1429–33. doi: 10.1016/j.clinbiochem.2011.08.1140. [DOI] [PubMed] [Google Scholar]
- 103.Lopez-Bonet E, Pérez-Martínez MC, Martin-Castillo B, Alonso-Ruano M, Tuca F, Oliveras-Ferraros C, et al. Diagnostic utility of mammaglobin and GCDFP-15 in the identification of primary neuroendocrine carcinomas of the breast. Breast Cancer Res Treat. 2011;126:241–5. doi: 10.1007/s10549-010-1229-9. [DOI] [PubMed] [Google Scholar]
- 104.Rollins-Raval M, Chivukula M, Tseng GC, Jukic D, Dabbs DJ. An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Arch Pathol Lab Med. 2011;135:975–83. doi: 10.5858/2009-0445-OAR2. [DOI] [PubMed] [Google Scholar]
- 105.Diel IJ, Kaufmann M, Costa SD, Holle R, von Minckwitz G, Solomayer EF, et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst. 1996;88:1652–8. doi: 10.1093/jnci/88.22.1652. [DOI] [PubMed] [Google Scholar]
- 106.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 107.Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14:2593–600. doi: 10.1158/1078-0432.CCR-07-4758. [DOI] [PubMed] [Google Scholar]
- 108.Tjensvoll K, Gilje B, Oltedal S, Shammas VF, Kvaløy JT, Heikkilä R, et al. A small subgroup of operable breast cancer patients with poor prognosis identified by quantitative real-time RT-PCR detection of mammaglobin A and trefoil factor 1 mRNA expression in bone marrow. Breast Cancer Res Treat. 2009;116:329–38. doi: 10.1007/s10549-008-0204-1. [DOI] [PubMed] [Google Scholar]
- 109.Zach O, Kasparu H, Wagner H, Krieger O, Lutz D. Prognostic value of tumour cell detection in peripheral blood of breast cancer patients. Acta Med Austriaca. 2002;59(Suppl):32–4. [PubMed] [Google Scholar]
- 110.Ferrucci PF, Rabascio C, Mazzetta C, Cocorocchio E, Agazzi A, Vanazzi A, et al. Mammaglobin expression in leukapheresis products is a predictive marker of poor prognosis in women with high-risk breast cancer. Clin Cancer Res. 2004;10:6039–46. doi: 10.1158/1078-0432.CCR-03-0453. [DOI] [PubMed] [Google Scholar]
- 111.Lin YC, Chen SC, Hsueh S, Lo YF, Chow-Wu YH, Liaw IC, et al. Lack of correlation between expression of human mammaglobin mRNA in peripheral blood and known prognostic factors for breast cancer patients. Cancer Sci. 2003;94:99–102. doi: 10.1111/j.1349-7006.2003.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marques AR, Teixeira E, Diamond J, Correia H, Santos S, Neto L, et al. Detection of human mammaglobin mRNA in serial peripheral blood samples from patients with non-metastatic breast cancer is not predictive of disease recurrence. Breast Cancer Res Treat. 2009;114:223–32. doi: 10.1007/s10549-008-0002-9. [DOI] [PubMed] [Google Scholar]
- 113.Nunez-Villar MJ, Martinez-Arribas F, Pollan M, Lucas AR, Sanchez J, Tejerina A, et al. Elevated mammaglobin (h-MAM) expression in breast cancer is associated with clinical and biological features defining a less aggressive tumour phenotype. Breast Cancer Res. 2003;5:R 65–70. doi: 10.1186/bcr587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bitisik O, Saip P, Saglam S, Derin D, Dalay N. Mammaglobin and maspin transcripts in blood may reflect disease progression and the effect of therapy in breast cancer. Genet Mol Res. 2010;9:97–106. doi: 10.4238/vol9-1gmr649. [DOI] [PubMed] [Google Scholar]
- 115.Bossolasco P, Ricci C, Farina G, Soligo D, Pedretti D, Scanni A, et al. Detection of micrometastatic cells in breast cancer by RT-PCR for the mammaglobin gene. Cancer Detect Prev. 2002;26:60–3. doi: 10.1016/s0361-090x(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 116.Li BJ, Wang JY, Wang HY, Huang XP, Zhang LJ, Long H, et al. [Clinical significance of hMAM mRNA detection in bone marrow of breast carcinoma patient] Zhonghua Zhong Liu Za Zhi. 2006;28:766–9. [PubMed] [Google Scholar]
- 117.Bharat A, Benshoff N, Fleming TP, Dietz JR, Gillanders WE, Mohanakumar T. Characterization of the role of CD8+T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res Treat. 2008;110:453–63. doi: 10.1007/s10549-007-9741-2. [DOI] [PubMed] [Google Scholar]
- 118.Ilias Basha H, Tiriveedhi V, Fleming TP, Gillanders WE, Mohanakumar T. Identification of immunodominant HLA-B7-restricted CD8+ cytotoxic T cell epitopes derived from ammaglobin-A expressed on human breast cancers. Breast Cancer Res Treat. 2011;127:81–9. doi: 10.1007/s10549-010-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tanaka Y, Amos KD, Fleming TP, Eberlein TJ, Goedegebuure PS. Mammaglobin-A is a tumor-associated antigen in human breast carcinoma. Surgery. 2003;133:74–80. doi: 10.1067/msy.2003.92. [DOI] [PubMed] [Google Scholar]
- 120.Zuo L, Li L, Wang Q, Fleming TP, You S. Mammaglobin as a potential molecular target for breast cancer drug delivery. Cancer Cell Int. 2009;23:9–8. doi: 10.1186/1475-2867-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, et al. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer. 2002;102:499–506. doi: 10.1002/ijc.10736. [DOI] [PubMed] [Google Scholar]
- 122.Jaramillo A, Narayanan K, Campbell LG, Benshoff ND, Lybarger L, Hansen TH, et al. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes by CD8+ cytotoxic T lymphocytes from breast cancer patients. Breast Cancer Res Treat. 2004;88:29–41. doi: 10.1007/s10549-004-8918-1. [DOI] [PubMed] [Google Scholar]
- 123.Manna PP, Jaramillo A, Majumder K, Campbell LG, Fleming TP, Dietz JR, et al. Generation of CD8+ cytotoxic T lymphocytes against breast cancer cells by stimulation with mammaglobin-A-pulsed dendritic cells. Breast Cancer Res Treat. 2003;79:133–6. doi: 10.1023/a:1023323509888. [DOI] [PubMed] [Google Scholar]
- 124.Narayanan K, Jaramillo A, Benshoff ND, Campbell LG, Fleming TP, Dietz JR, et al. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J Natl Cancer Inst. 2004;96:1388–96. doi: 10.1093/jnci/djh261. [DOI] [PubMed] [Google Scholar]
- 125.Tiriveedhi V, Sarma NJ, Subramanian V, Fleming TP, Gillanders WE, Mohanakumar T. Identification of HLA-A24-restricted CD8(+) cytotoxic T-cell epitopes derived from mammaglobin-A, a human breast cancer-associated antigen. Hum Immunol. 2012;73:11–6. doi: 10.1016/j.humimm.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viehl CT, Frey DM, Phommaly C, Chen T, Fleming TP, Gillanders WE, et al. Generation of mammaglobin-A-specific CD4 T cells and identification of candidate CD4 epitopes for breast cancer vaccine strategies. Breast Cancer Res Treat. 2008;109:305–14. doi: 10.1007/s10549-007-9657-x. [DOI] [PubMed] [Google Scholar]
- 127.Viehl CT, Tanaka Y, Chen T, Frey DM, Tran A, Fleming TP, et al. Tat mammaglobin fusion protein transduced dendritic cells stimulate mammaglobin-specific CD4 and CD8 T cells. Breast Cancer Res Treat. 2005;91:271–8. doi: 10.1007/s10549-005-0450-4. [DOI] [PubMed] [Google Scholar]
- 128.Goedegebuure PS, Watson MA, Viehl CT, Fleming TP. Mammaglobin-based strategies for treatment of breast cancer. Curr Cancer Drug Targets. 2004;4:531–42. doi: 10.2174/1568009043332862. [DOI] [PubMed] [Google Scholar]
- 129.Shi CX, Long MA, Liu L, Graham FL, Gauldie J, Hitt MM. The human SCGB2A2 (mammaglobin-1) promoter/enhancer in a helper-dependent adenovirus vector directs high levels of transgene expression in mammary carcinoma cells but not in normal nonmammary cells. Mol Ther. 2004;10:758–67. doi: 10.1016/j.ymthe.2004.06.849. [DOI] [PubMed] [Google Scholar]
- 130.Baxevanis CN, Voutsas IF, Gritzapis AD, Perez SA, Papamichail M. HER-2/neu as a target for cancer vaccines. Immunotherapy. 2010;2:213–26. doi: 10.2217/imt.09.89. [DOI] [PubMed] [Google Scholar]
- 131.Koh EH, Cho YW, Mun YJ, Ryu JH, Kim EJ, Choi DS, et al. Upregulation of human mammaglobin reduces migration and invasion of breast cancer cells. Cancer Invest. 2014;32:22–9. doi: 10.3109/07357907.2013.861473. [DOI] [PubMed] [Google Scholar]


