Figure 1.
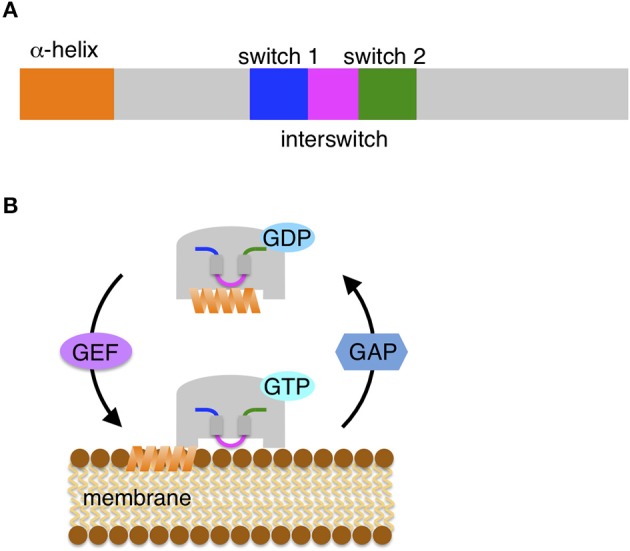
Small GTPase Sar1/Arf1 protein. (A) Schematic diagrams of Sar/Arf. Conserved domains are depicted: the N-terminal amphipathic α-helix, two switch regions (switch 1 and switch 2) and the interswitch region. (B) The Sar/Arf protein cycles between membrane-association and dissociation. GDP-bound cytosolic Sar/Arf is inactive and carries the N-terminal amphipathic helix in a hydrophobic pocket. A guanine nucleotide exchange factor (GEF) mediates the exchange of GDP for GTP in Sar/Arf. GTP-loaded Sar/Arf undergoes a conformational change of the two switch and interswitch regions, triggering the extrusion of the helix from the pocket. Subsequently, the shallow insertion of the amphipathic helix into the outer leaflet of the lipid bilayers allows Sar/Arf to associate tightly with the membrane surface. For dissociation, GTPase activating protein (GAP) activates the GTP hydrolysis activity of Sar/Arf. Conserved domains are shown in the same color in (A) and (B).
