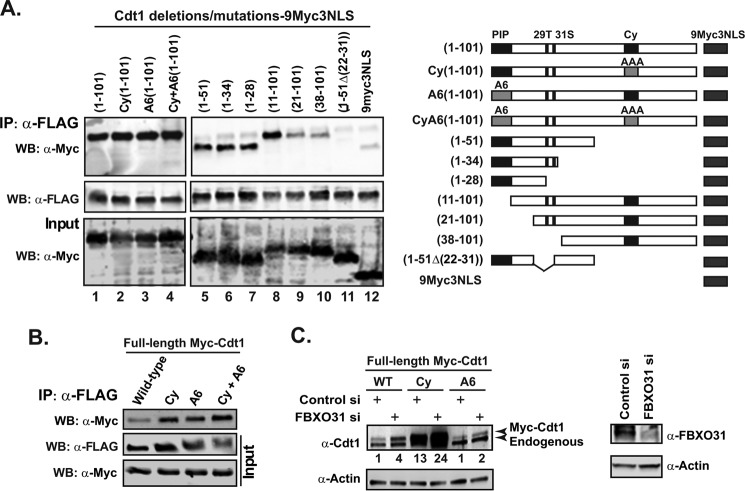
FIGURE 4.
FBXO31 interacts with Cdt1 N terminus and stabilizes Cdt1 Cy and A6 mutants. A, HEK293T cells stably expressing FLAG-FBXO31 were transfected with Cdt1 N-terminal constructs fused to 9myc3NLS. Cell lysates were IP with an anti-FLAG antibody. Input and IP samples were Western-blotted (WB) with anti-Myc or anti-FLAG antibodies. A schematic of the Cdt1 N-terminal constructs is illustrated below the figure. The 29T amino acid in the Cdt1 protein is a phosphorylation target closely located to Ser-31, which is also a potential phosphorylation target (47). B, HEK293T cells stably expressing FLAG-FBXO31 were transfected with constructs expressing full-length Myc tagged Cdt1 proteins (wild type, Cy, A6, or CyA6). Cell lysates were immunoprecipitated with an anti-FLAG antibody. Input and IP samples were Western-blotted with anti-Myc or anti-FLAG antibodies. C, HeLa cells were first transfected with control or FBXO31 siRNA (siRNA-2) on day 1, and then a control or FBXO31 siRNA set was transfected with either wild type, Cy, or A6-Cdt1 expression constructs on day 2. The cells were harvested 48 h post-transfection and analyzed by Western blotting with anti-Cdt1, anti-FBXO31, and anti-actin antibodies. The Myc-Cdt1 protein levels were quantified using the ImageJ software and normalized against actin.