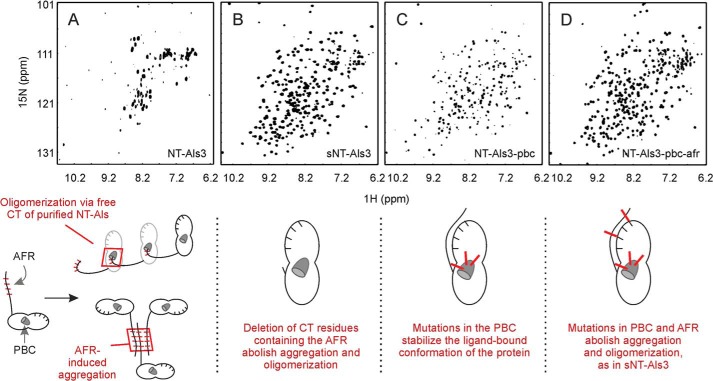
FIGURE 2.
NMR spectra (1H-15N TROSY-HSQC) showing the effect of mutations in the PBC and AFR on NT-Als3. A, the NMR spectrum of purified NT-Als3 showed considerably reduced solubility. Two potential mechanisms explain this effect: oligomerization caused by insertion of the free C terminus (CT) of the protein as a ligand into the binding pocket of an adjacent NT-Als3 molecule (“self-complementation,” left) (14) and aggregation mediated by exposed AFR sequences (30). Note that self-complementation is suppressed in full-length Als proteins on the C. albicans surface, where the C terminus is linked to the cell wall via a glycosylphosphatidylinositol anchor remnant (10). B, deletion of the AFR resulted in sNT-Als3, a construct free of oligomerization and aggregation effects. C, mutations in the PBC (K59M, A116V, and Y301F to generate NT-Als3-pbc) improved protein solubility compared with A, presumably due to elimination of self-complementation and decreased aggregation because the AFR was associated with the rest of the NT-Als3 folded structure (Fig. 3). D, solubility of the protein in C was further improved by removing residual AFR activity via mutations I311S/I313S (NT-Als3-pbc-afr). These results demonstrate at the molecular level the role of the PBC and the AFR in aggregation among purified NT-Als proteins.