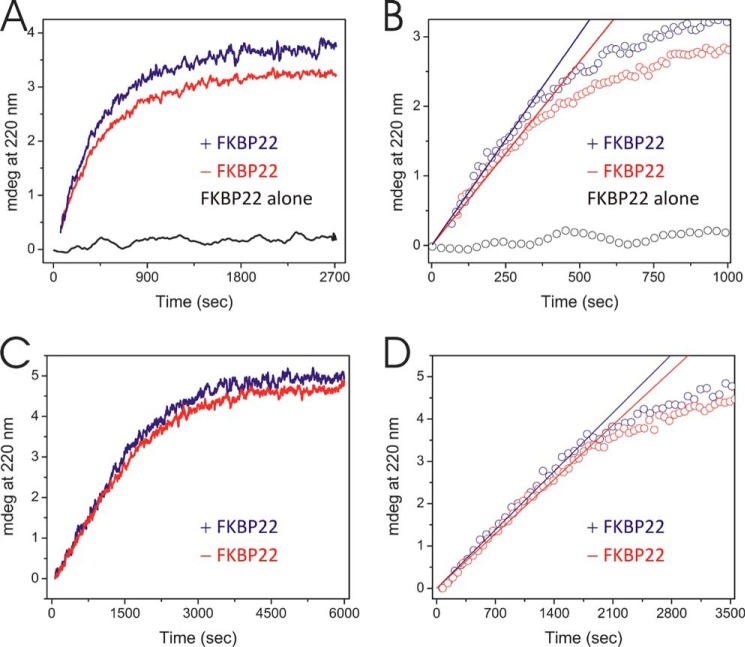
FIGURE 4.
Refolding of the quarter fragment of type III collagen with and without prolyl 4-hydroxylation in the presence and absence of FKBP22. Refolding was monitored by a circular dichroism spectrum at 220 nm. Protein concentrations were 2 and 6 μm for the quarter fragments of type III collagens and FKBP22, respectively. A, refolding of the prolyl 4-hydroxylated quarter fragment of type III collagen in the presence (blue) and absence (red) of FKBP22. The black curve represents FKBP22 by itself. B, determination of the initial folding rate of prolyl 4-hydroxylated quarter fragment of type III collagen in the presence (blue) and absence (red) of FKBP22. Open circles and solid straight lines, raw data points and calculated initial folding rate from A, respectively. The slope of the straight lines reflected the initial rate of folding of prolyl 4-hydroxylated quarter fragment of type III collagen. Open black circles, raw data points of FKBP22 alone. C, refolding of non-4-hydroxylated quarter fragment of type III collagen in the presence (blue) and absence (red) of FKBP22. D, the determination of initial folding kinetics of the non-4-hydroxylated quarter fragment of type III collagen in presence (blue) and absence (red) of FKBP22. Open circles and solid straight lines, raw data points and calculated initial folding rate from C, respectively. The slope of the straight line reflected the initial rate of folding of the non-hydroxylated quarter fragment of type III collagen.