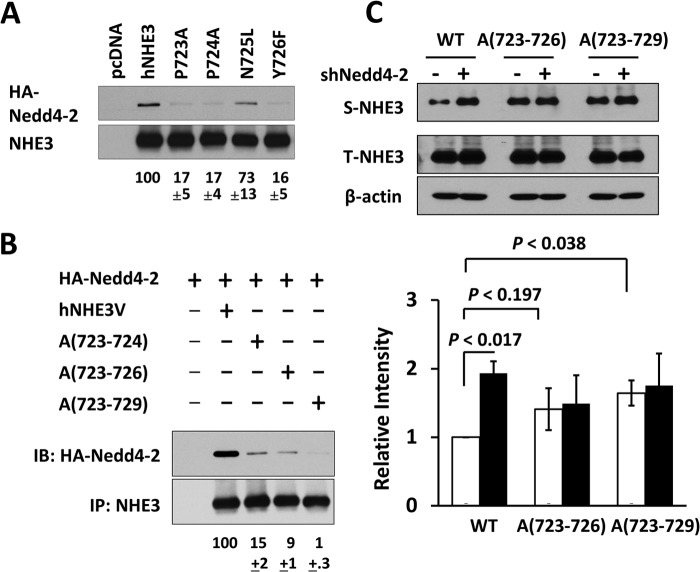
FIGURE 7.
The extended PY motif of hNHE3 mediates interaction with Nedd4-2. A, each of the amino acid residues in the PY motif in hNHE3 was changed to Ala, and the interaction with HA-Nedd4-2 was determined by co-immunoprecipitation in PS120 cells. The extent of interaction with Nedd4-2 was quantified relative to full-length hNHE3. B, the following mutations were made: PP of PPNY to Ala (A(723–724)); PPNY to Ala (Ala-(723–726)); PPNYDEE to Ala (Ala-(723–729)). The interaction with Nedd4-2 was determined as described above. IB, immunoblot. C, the effect of Nedd4-2 silencing was determined on surface expression of Ala-(723–726) and Ala-(723–729) in PS120 cells transfected with shNedd4-2 (+) or shCon (−). Representative figures from three independent experiments are shown. The bar graph shows quantification of S-NHE3/T-NHE3 in each sample normalized to WT transfected with shCon. Open bar, shCon; closed bar, shNedd4-2. n = 3.