FIGURE 6.
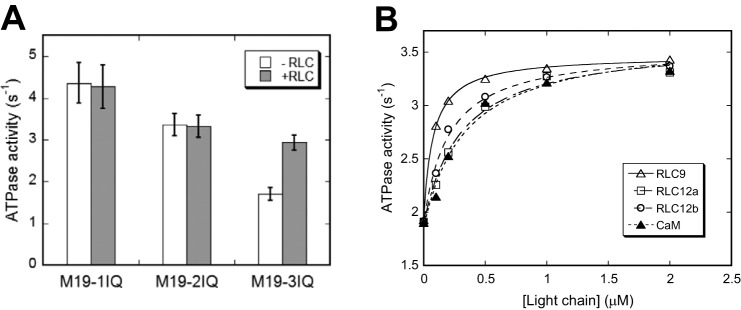
Exogenous light chain enhances actin-activated ATPase activity of M19–3IQ. A, actin-activated ATPase activity of Myo19-truncated constructs in the presence or absence of 4 μm RLC9 and RLC12b. Values are mean ± S.D. from two to four independent assays. B, effects of exogenous light chain on actin-activated ATPase activity of M19-3IQ. ATPase assay was performed in 50 mm NaCl, 20 mm MOPS (pH 7.0), 1 mm MgCl2, 1 mm EGTA, 0.25 mg/ml of BSA, 1 mm DTT, 0.5 mm ATP, 2.5 mm phosphoenol pyruvate, 20 units/ml of pyruvate kinase, 40 μm actin, and 0–2 μm light chain. The stimulation of ATPase activities by exogenous light chain was fitted with a hyperbolic equation: V = V0 + Vmax × [LC]/(Kd + [LC]), where V0, the activity in the absence of exogenous light chain; Vmax, the activity stimulated by exogenous light chain; [LC], the concentration of exogenous light chain; Kd, the apparent affinity between the light chain and M19-IQ3. The apparent Kd values are 0.076 ± 0.027, 0.321 ± 0.076, 0.223 ± 0.068, and 0.335 ± 0.049 μm for RLC9, RLC12a, RLC12b, and CaM, respectively. Data were average of two independent assays.
