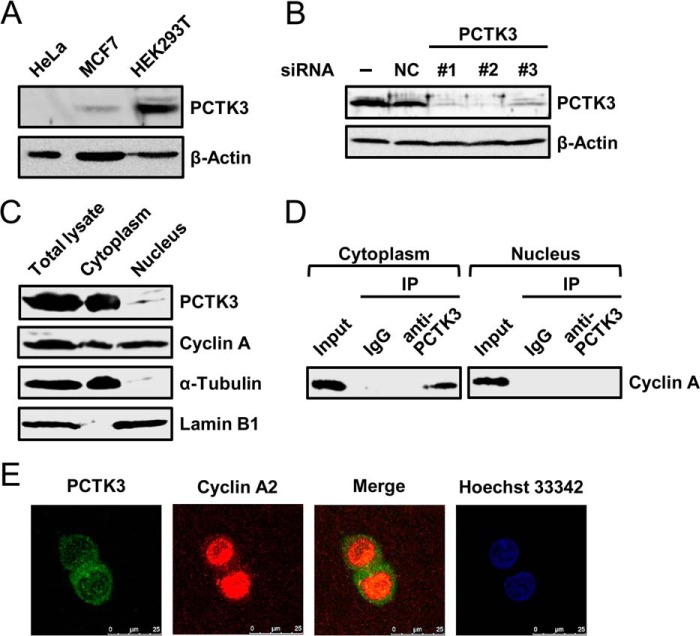
FIGURE 3.
Endogenous interaction between PCTK3 and cyclin A in the cytoplasm. A, immunoblot analysis of total proteins prepared from HeLa, MCF7, and HEK293T human cell lines. β-Actin was used as a loading control. B, siRNA knockdown of PCTK3 in HEK293T cells. Cells were transfected with three different siRNAs against PCTK3 (#1–3) or negative control siRNA (NC). Forty-eight hours after transfection, cell extracts were immunoblotted with anti-PCTK3 antibody. Expression of β-actin was used as a loading control. C, subcellular distributions of endogenous PCTK3 and cyclin A. HEK293T cells were separated into nuclear and cytoplasmic fractions as described under “Experimental Procedures.” Aliquots of each fraction were immunoblotted for PCTK3 and cyclin A. The purity of nuclear and cytoplasmic fractions was confirmed using anti-lamin B1 antibody as a nuclear marker and α-tubulin antibody as a cytoplasmic marker, respectively. D, endogenous interaction between PCTK3 and cyclin A in HEK293T cells. The fractionated lysates from HEK293T cells were immunoprecipitated (IP) using anti-PCTK3 antibody. The immunoprecipitates were analyzed by immunoblotting with anti-cyclin A antibody. Normal rabbit IgG (IgG) was used as a control. E, intracellular localization of endogenous PCTK3. HEK293T cells were fixed and incubated with rabbit anti-PCTK3 and mouse anti-cyclin A antibodies. The primary antibody was visualized with Alexa Fluor 488-conjugated anti-rabbit IgG and Alexa Fluor 555-conjugated anti-mouse IgG followed by confocal microscopy. Fluorescence for PCTK3 (green) and cyclin A (red) is shown with the merged images (merge is in yellow). Hoechst nuclear staining is represented in blue.