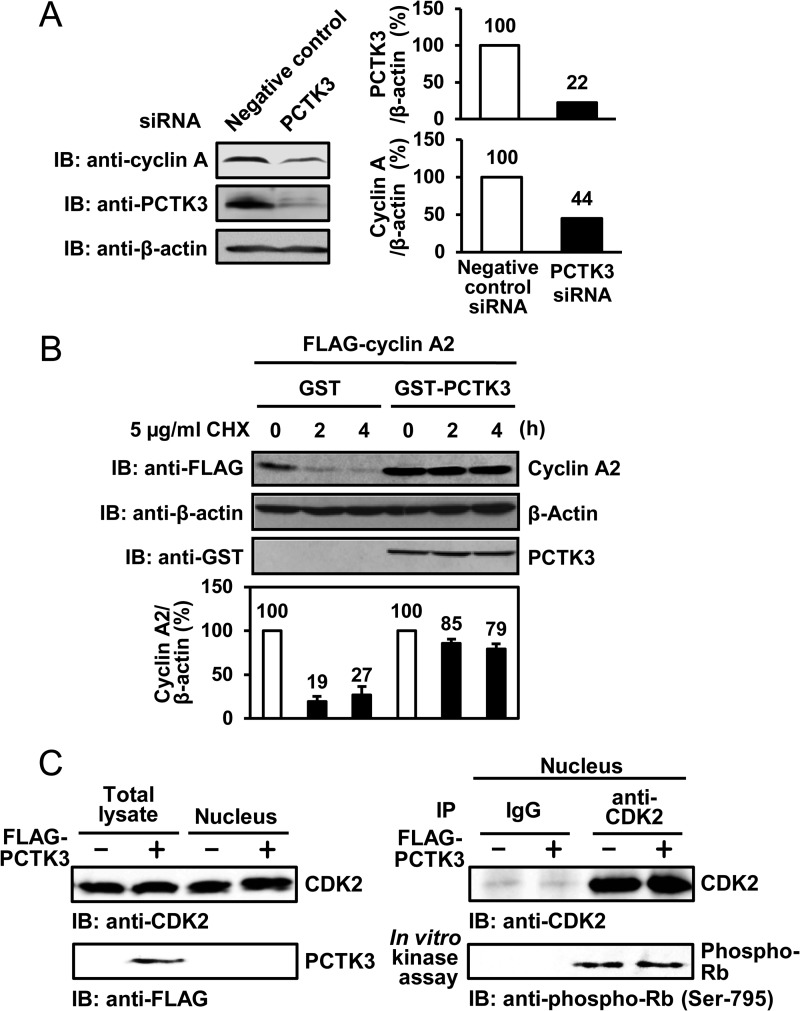
FIGURE 5.
PCTK3 protects cyclin A2 against degradation in the cytoplasm. A, HEK293T cells were transfected with PCTK3 siRNA. The cell lysates were subjected to immunoblotting (IB) with anti-PCTK3 and anti-cyclin A antibodies. Expression of α-tubulin was used as a loading control. B, HEK293T cells were cotransfected with GST or GST-PCTK3 together with FLAG-cyclin A2. After 24 h, cycloheximide (CHX) (5 μg/ml) was added to the cell culture. Cells were harvested at indicated times after cycloheximide treatment and FLAG-cyclin A2 protein levels were determined by immunoblotting (IB: anti-FLAG). β-Actin was used as a loading control (IB: anti-β-actin). FLAG-cyclin A2 protein levels were quantified by densitometric analysis and represented in a graph. C, HEK293T cells were transfected with FLAG-PCTK3 and separated into nuclear and cytoplasmic fractions. The nuclear fractions were immunoprecipitated (IP) using anti-CDK2 antibody, and the immunoprecipitates were used in an in vitro kinase assay with MBP-Rb C as a substrate. Normal rabbit IgG (IgG) was used as a control.