Abstract
Physical activity is recommended for skeletal health because bones adapt to mechanical loading. The young skeleton shows greatest plasticity to physical activity-related mechanical loads, but bones are most at risk of failure later in life. The discrepancy raises the question of whether the skeletal benefits of physical activity completed when young persist with aging. Here we present a unique case wherein the cortical bone benefit of physical activity completed over five decades earlier could be established within an individual aged in their tenth decade of life. Specifically, we compared bone properties at the midshaft humerus between the throwing and nonthrowing arms of a 94-year-old former Major League Baseball player who ceased throwing 55 years earlier. By performing analyses within-subject, the long-term skeletal benefit of physical activity completed when young could be assessed independent of inherited and systemic traits. Also, as the subject threw left-handed during his throwing career, but was right-hand dominant in all other activities throughout life, any lasting skeletal benefits in favor of the throwing arm could not be attributable to simple arm dominance. Analyses indicated that any cortical bone mass, area and thickness benefits of throwing-related physical activity completed when young were lost with aging, possibly due to accelerated intracortical remodeling. In contrast, the subject’s throwing (nondominant) arm had greater total cross-sectional area and estimated strength (polar moment of inertia) than in his dominant arm, despite muscle indices favoring the latter. These data indicate that physical activity completed when young can have lasting benefits on bone size and strength, independent of the maintenance of bone mass benefits.
Keywords: bone size, bone structure, exercise, mechanical loading, osteoporosis
Introduction
Physical activity when young is advocated for lifelong bone health, with the premise being that achieving a higher peak bone mass during youth may prime the skeleton to offset the bone loss associated with aging [1]. Prospective observational studies suggest that some of the bone mass benefits generated through physical activity when young may persist into early adulthood [2–5], and a recent study with a mean follow-up of 39 years provided evidence to suggest benefits may last into later adulthood [6]. However, the latter study did not completely control for selection bias, with inherited traits possibly explaining the greater bone mass in former athletes compared to controls at both baseline and follow-up. To assess the long-term skeletal benefit of physical activity completed during youth while controlling for inherited and other systemic traits, we recently used a within-subject controlled model comparing the throwing and nonthrowing arms in former professional (Major League Baseball [MLB]) throwing athletes [7]. Overhand throwing loads the humerus and induces substantial adaptation [7–11]. Throwing-to-nonthrowing arm differences in the former MLB players were compared to dominant-to-nondominant differences in control subjects to isolate the skeletal benefits of throwing-related physical activity from effects due to elevated habitual unilateral loading associated with arm dominance. Data showed that the benefits of throwing-related physical activity on dual-energy x-ray absorptiometry (DXA)-derived measures of upper extremity bone mineral content (BMC) were lost within 30–39 years following activity cessation [7]—an observation consistent with the eventual loss of lower extremity bone mass benefits of physical activity observed in a prominent study of former soccer players [12].
The eventual loss of the bone mass benefits of physical activity completed when young makes evolutionary sense considering that humans evolved for endurance [13] and it is energy inefficient to maintain skeletal mass in excess of prevailing needs. However, it is possible that the bone size benefits of physical activity completed when young persist long-term and contribute to lasting benefits on bone strength. Physical activity prior to mid-adolescence preferentially deposits new bone on the periosteal surface resulting in an increase in bone size [14,15]. As bone mechanical properties are proportional to the fourth power of the bone radius [16] and bone loss during aging occurs primarily near the endocortical surface and not the periosteal surface [17], the enhanced bone size generated by physical activity when young may remain intact until senescence to have anti-fracture benefits. We previously demonstrated mechanical loading when young conferred lifelong benefits in bone size and strength in rodent models [18–20]. Similarly, in our recent study involving former MLB players, over half of the bone size and one-third of the estimated bone strength benefits of throwing-related physical activity completed during youth persisted until the ninth decade of life [7]. The current report furthers our recent work by presenting a unique case wherein the cortical bone benefit of physical activity completed when young could be established within an individual aged in their tenth decade of life.
Case Report
A 94-year-old former MLB player was recruited as part of our larger study exploring the long-term benefits of physical activity on cortical bone properties [7]. The subject was not eligible to participate in the larger study because his throwing arm and dominant arm were not on the same side of the body negating the ability to pool his data with other former throwers—the subject threw left-handed during his throwing career due to a self-reported lack of left-handed pitchers during his era of play, but was right-hand dominant in all other activities throughout life. The contrasting throwing vs. dominant arm scenario provided a unique opportunity to establish the lasting skeletal benefit of throwing-related physical activity when young within the oldest subject in our cohort as any benefits in bone mass, size or estimated strength in favor of the throwing arm could not be attributable to arm dominance.
Demographic and anthropometric characteristics of the case subject are detailed in Table 1. The subject was in extremely good health considering his age, with the only regular medication being a low-dose thyroid tablet for long-standing mild hypothyroidism. He had not participated more than once per month for longer than 6 months in any throwing or other one-arm dominant activity (including vocation) in the 55 years since retiring from professional throwing meaning the lasting skeletal benefits of physical activity completed when young could be explored long-term following return to habitual loading levels. All procedures were performed with approval of the Institutional Review Board of Indiana University and following obtainment of written informed consent from the subject.
Table 1.
Case subject demographic and anthropometric characteristics
| Characteristic | |
|---|---|
| Demographics | |
| Age (yr) | 94.3 |
| Age started recreational throwing (yr) | 6 |
| Age started competitive throwing (yr) | 9 |
| Estimated age of adolescent growth spurt (yr) | 14 |
| Years throwing before adolescent growth spurt (yr) | 8 |
| Age started professional (MiLB/MLB) baseball (yr) | 18 |
| Professional (MiLB/MLB) baseball games played (n) | 1776 |
| Age ceased throwing (yr) | 39 |
| Total years throwing (yr) | 33 |
| Years detraining (yr) | 55 |
| Whole-body anthropometry | |
| Height (m) | 1.74 |
| Mass (kg) | 66.9 |
| BMI (kg/m2) | 22.1 |
| aBMD (g/cm2)† | 1.27 |
| Lean mass (kg)† | 48.0 |
| Fat mass (%)† | 23.2 |
| Regional anthropometry | |
| Spine aBMD (g/cm2) [T-score]† | 1.08 [−0.1] |
| Hip aBMD (g/cm2) [T-score]† | 0.93 [−0.7] |
MiLB/MLB = Minor/Major League Baseball; BMI = body mass index; aBMD = areal bone mineral density
Obtained using dual-energy x-ray absorptiometry
Peripheral quantitative computed tomography (pQCT) (XCT 3000; Stratec Medizintechnik GmbH) was performed to assess bone volumetric density, mass, size and estimated strength at the level of the midshaft humerus, using previously described procedures [7,11]. The midshaft humerus was chosen as the site of interest as it displays substantial adaptation to the mechanical loads associated with overhand throwing, as observed by greater cortical BMC, area and thickness, reduced medullary area, and greater total area/size and estimated strength [7,11]. DXA (Discovery-W; Hologic, Inc.) for whole-body (including bilateral upper extremities) BMC and composition, and spine and hip BMC was also performed.
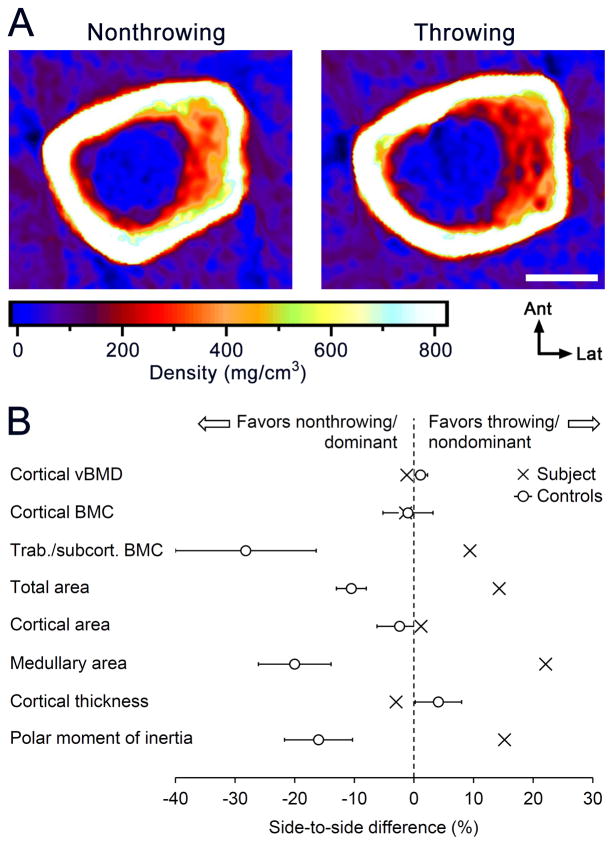
pQCT images of the midshaft humerus in the throwing/nondominant and nonthrowing/dominant arms of the subject are shown in Figure 1A. The throwing/nondominant arm had 1.4% less cortical BMC at the midshaft humerus than in the nonthrowing/dominant arm (Fig. 1B). The small difference in favor of the dominant arm approximated the dominant-to-nondominant difference observed in a cohort of controls (n=11; age = 83.8 ± 2.5 years) (Fig. 1B). The controls had not participated more than once per month for longer than 6 months in any athletic activity or vocation that preferentially utilized one arm, and were tested to explore side-to-side differences attributable to simple arm dominance as part of our larger study [7]. The result suggests loss of the bone mass benefits induced by throwing-related physical activity completed when young, with currently active MLB/Minor League Baseball (MiLB) players having 50.4% (95%CI, 44.2 to 56.6%) greater midshaft humerus cortical BMC in their throwing arm compared to their nonthrowing arm [7]. Regional analyses of whole-body DXA scans confirmed the loss of the bone mass benefit of physical activity completed when young in the subject, with whole-arm (humerus, radius, ulna, carpal and hand) BMC in the throwing/nondominant arm being 4.1% less than in the nonthrowing/dominant arm. Whole-arm BMC in favor of the subject’s dominant arm matches the 7.1% (95%CI, 1.8 to 12.3%) greater whole-arm BMC observed in controls due to arm dominance, and contrasts the 30.5% (95%CI, 25.9 to 35.1%) greater whole-arm BMC observed in the throwing arm compared to the nonthrowing arm in currently active MLB/MiLB players [7].
Fig. 1.
Physical activity-related mechanical loading when young had long-term benefits on cortical bone size and estimated strength, but not cortical bone mass. (A) Peripheral QCT images of the midshaft humerus in the nonthrowing/dominant arm and throwing/nondominant arm of the case subject. Note the greater overall bone cross-sectional size and medullary area in the throwing arm, but equivalent cortical thickness despite it being the subject’s nondominant arm. Scale bar represents 20 mm. (B) Mean difference between the throwing arm and nonthrowing arm of the case subject (indicated by X’s) for bone density, mass, structure, and estimated strength. Data for the mean percent difference and 95% confidence interval between the dominant arm and the nondominant arm in controls (n=11; age=83.8 ± 2.5 years) from [7] are shown for comparison.
The loss of the bone mass benefits of physical activity completed when young was coupled with loss of any cortical area and thickness benefits (Fig. 1A, B), and appeared to result from greater medullary expansion and cortical trabecularization in the throwing arm relative to the nonthrowing arm during aging. Accelerated medullary expansion was evidenced by 22.1% greater medullary area in the throwing/nondominant arm relative to the nonthrowing/dominant arm, whereas 9.4% greater trabecular/subcortical bone mineral content in the throwing/nondominant arm provided evidence of accelerated cortical trabecularization (Fig. 1A, B). The greater medullary area and trabecular/subcortical bone mineral content in the subject’s throwing/nondominant arm contrasts the side-to-side differences in favor of the dominant arm observed in controls (Fig. 1B), and contrasts the 17.1% (95% CI, 5.0 to 29.2%) smaller medullary area observed in the throwing arm compared to the nonthrowing arm in currently active MLB/MiLB players [7].
Although the cortical bone mass, area and thickness benefits of throwing-related physical activity completed when young appeared to be lost with aging, a portion of the bone size and estimated strength benefits persisted. Despite being the subject’s nondominant arm, the midshaft humerus in his throwing arm had 14.3% greater total cross-sectional area/size and 15.2% greater estimated torsional rigidity (as indicated by the density-weighted polar moment of inertia) than in his nonthrowing arm (Fig. 1A, B). The side-to-side differences in favor of the subject’s throwing/nondominant arm contrasts the greater total area and torsional rigidity in favor of the dominant arm in controls (Fig. 1B), indicating that throwing-related physical activity completed when young had lasting benefits on bone size and strength in the subject that withstood the influence of elevated habitual loading associated with arm dominance.
The lasting benefit of throwing-related physical activity in bone size and strength within the throwing arm of the subject also persisted independent of side-to-side differences in muscle measures. DXA-derived upper extremity lean mass and pQCT-derived upper arm muscle cross-sectional area were 19.2% (2.38 vs. 2.95 kg) and 10.5% (23.9 vs 26.7 cm2) less in the throwing/nondominant arm relative to the nonthrowing/dominant arm. The differences in favor of the dominant arm approximate those observed due to arm dominance in controls where upper extremity lean mass (15.6% [95%CI, 10.6 to 20.5%]) and upper arm muscle cross-sectional area (2.6% [95%CI, 0.1 to 5.2%]) also favored the dominant arm [7].
Discussion
The current case provides evidence suggesting that physical activity has benefits on bone size and estimated strength, but not mass when assessed in the tenth decade of life and more than five decades after activity completion. The age of the subject exceeds that of our previous work [7], enabling the case to advance knowledge and provide a unique contribution. The ability to establish the lasting skeletal benefits of physical activity within a single subject independent of inherited traits, systemic factors, and arm dominance was discussed in preceding sections.
The apparent loss of any cortical BMC, area and thickness benefits of physical activity completed when young in the subject are consistent with findings in our larger study [7], and suggest that cessation of throwing-related physical activity was associated with accelerated age-related bone loss and structural decay. Age-related cortical bone loss is principally characterized by intracortical remodeling within the cortex adjacent to the medullary cavity which thins the cortex from within by cavitation and leaves cortical remnants that look similar to trabeculae [21]. Accelerated intracortical remodeling in the current case appeared evident by greater medullary area/expansion and characterization of cortical bone material as trabecular/subcortical in the throwing arm relative to nonthrowing arm.
Although accelerated intracortical remodeling appeared to effectively remove any bone mass benefits of physical activity completed when young, it did not remove the bone size benefits. The subject’s throwing arm persisted to have a larger total cross-sectional area at the midshaft humerus compared to his nonthrowing arm despite the latter being his dominant arm and having greater measures of muscle properties. The lasting bone size benefit fits our working hypothesis that the general absence of age-related bone loss on the periosteal surface (and actual expansion of this surface during aging [22,23]) enables the bone size benefits of physical activity completed when young to persist long-term. The lasting bone size benefit observed in the subject extends findings from our larger study where over 50% of the size benefit of throwing-related physical activity completed when young was maintained into the ninth decade of life [7]. In addition, it furthers the recent findings of Nilsson et al. [24] who cross-sectionally compared individuals in quartiles of previous physical activity levels to report lasting bone size benefits of physical activity in the tibia.
The ultimate outcome of maintenance of some of the benefit of physical activity completed when young on bone size was maintenance of some of the estimated strength benefit. As indicated earlier, bone mechanical properties are proportional to the fourth power of the bone radius [16]. By having the same amount of bone material as the nonthrowing arm but distributing it further from the central axis, the midshaft humerus in the subject’s throwing arm was estimated to be stronger than in his contralateral nonthrowing arm.
Ultimately, these data indicate that physical activity completed when young can have lasting benefits on bone size and strength when assessed in the tenth decade of life and five decades following the return to habitual levels of loading. Whether the observed benefits persist in women and at sites prone to osteoporotic fracture remains to be established. It may be that physical activity targeting sites prone to osteoporotic fracture needs to be continued during aging so as to reduce the accelerated intracortical bone remodeling observed with complete return to habitual loading.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant R01 AR057740 (to S.J.W.)
References
- 1.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Faulkner RA, et al. Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: a 14-year follow-up. J Bone Miner Res. 2012;27:104–110. doi: 10.1002/jbmr.514. [DOI] [PubMed] [Google Scholar]
- 3.Kontulainen S, Kannus P, Haapasalo H, Sievanen H, Pasanen M, et al. Good maintenance of exercise-induced bone gain with decreased training of female tennis and squash players: a prospective 5-year follow-up study of young and old starters and controls. J Bone Miner Res. 2001;16:195–201. doi: 10.1359/jbmr.2001.16.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Pollock NK, Laing EM, Modlesky CM, O’Connor PJ, Lewis RD. Former college artistic gymnasts maintain higher BMD: a nine-year follow-up. Osteoporos Int. 2006;17:1691–1697. doi: 10.1007/s00198-006-0181-3. [DOI] [PubMed] [Google Scholar]
- 5.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011;22:2205–2210. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tveit M, Rosengren BE, Nilsson JA, Ahlborg HG, Karlsson MK. Bone mass following physical activity in young years: a mean 39-year prospective controlled study in men. Osteoporos Int. 2013;24:1389–1397. doi: 10.1007/s00198-012-2081-z. [DOI] [PubMed] [Google Scholar]
- 7.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111:5337–5342. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop Relat Res. 1969;67:116–123. [PubMed] [Google Scholar]
- 9.Neil JM, Schweitzer ME. Humeral cortical and trabecular changes in the throwing athlete: a quantitative computed tomography study of male college baseball players. J Comput Assist Tomogr. 2008;32:492–496. doi: 10.1097/RCT.0b013e31811ec72d. [DOI] [PubMed] [Google Scholar]
- 10.Bogenschutz ED, Smith HD, Warden SJ. Mid-humerus adaptation in fast pitch softballers and the impact of throwing mechanics. Med Sci Sports Exerc. 2011;43:1698–1706. doi: 10.1249/MSS.0b013e3182134e4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warden SJ, Bogenschutz ED, Smith HD, Gutierrez AR. Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone. 2009;45:931–941. doi: 10.1016/j.bone.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, et al. Exercise during growth and bone mineral density and fractures in old age. Lancet. 2000;355:469–470. doi: 10.1016/s0140-6736(00)82020-6. [DOI] [PubMed] [Google Scholar]
- 13.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 14.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 15.Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: Ontogeny. Am J Phys Anthropol. 1994;93:35–54. doi: 10.1002/ajpa.1330930103. [DOI] [PubMed] [Google Scholar]
- 16.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 17.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 18.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22:251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 19.Warden SJ, Galley MR, Hurd AL, Richard JS, George LA, et al. Cortical and trabecular bone benefits of mechanical loading are maintained long-term in mice independent of ovariectomy. J Bone Miner Res. 2014;29:1131–1140. doi: 10.1002/jbmr.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warden SJ, Galley MR, Hurd AL, Wallace JM, Gallant MA, et al. Elevated mechanical loading when young provides lifelong benefits to cortical bone properties in female rats independent of a surgically induced menopause. Endocrinology. 2013;154:3178–3187. doi: 10.1210/en.2013-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zebaze RMD, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 22.Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, et al. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–408. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, Sundh D, Ohlsson C, Karlsson M, Mellstrom D, et al. Exercise during growth and young adulthood is independently associated with cortical bone size and strength in old Swedish men. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2212. in press. [DOI] [PubMed] [Google Scholar]