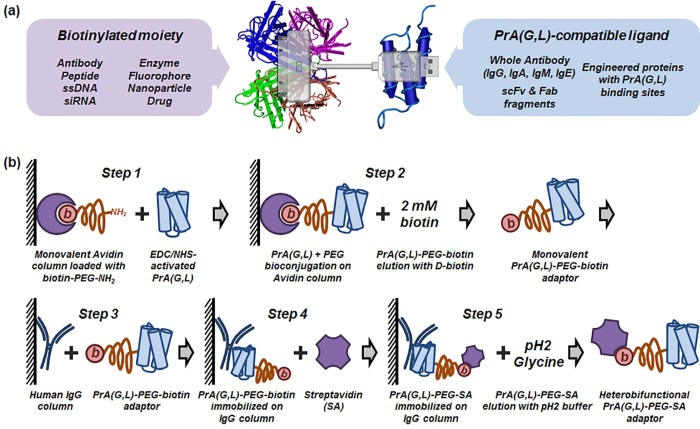
Figure 1.
Preparation of universal molecular adaptors for bispecific ligand self-assembly. (a) Schematic of a universal heterobifunctional adaptor molecule consisting of Streptavidin and Protein A(G,L) connected via PEG linker. Use of two versatile binary affinity systems—SA/biotin and PrA(G,L)/Antibody—enables assembly of a wide variety of ligands in a simple mix-and-use manner. Streptavidin and Protein A Z-domain structures adopted from NCBI MMDB. (b) Workflow of solid-phase bioconjugation of heterobifunctional PrA(G,L)-PEG-SA adaptors. Monomeric avidin column (with free amines blocked) is loaded with biotin-PEG-NH2. Following avidin–biotin binding, PrA(G,L) activated by EDC/NHS is added and allowed to conjugate to primary amine groups on PEG. Physical separation of conjugation sites (NH2 groups) ensures conjugation of PrA(G,L) to only one PEG molecule, yielding 1:1 PrA(G,L):PEG–biotin conjugates. Monofunctional PrA(G,L)-PEG-biotin is eluted with 2 mM d-biotin, further immobilized onto IgG agarose column and incubated with excess SA. Similarly, physical separation of biotin moieties on a solid surface ensures SA binding to only one biotin, forming column-bound heterobifunctional PrA(G,L)-PEG-SA adaptors. Finally, elution with 0.1 M pH 2.4 Glycine releases fully functional product from the column.