Figure 5.
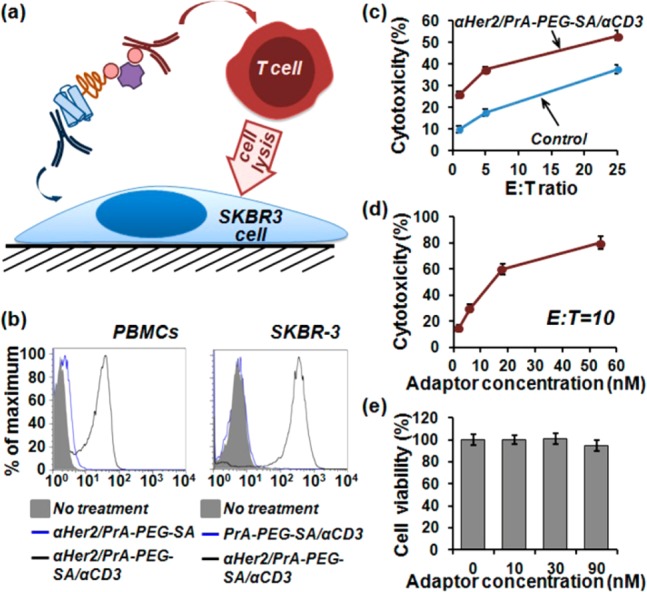
Assembly of bispecific CD3xHer2 antibodies via PrA-PEG-SA adaptors for enhanced T cell-mediated lysis of cancer cells. (a) Schematic of T cell-mediated lysis of cancer cells directed via cross-linking with self-assembled bispecific antibodies. (b) Characterization of target-binding functionality of CD3xHer2 bispecific antibodies with flow cytometry. Both CD3-positive PBMCs and Her2-positive SKBR3 cells were specifically labeled with bispecific antibodies, whereas ligands missing a single targeting antibody failed to bind to respective cells. (c) PBMCs (effector, E) were co-cultured with SKBR3 cells (target, T) at different E:T ratios in the presence of 10 nM CD3xHer2 ligands or equal amount of control (mixed anti-CD3 and anti-Her2 antibodies), exhibiting enhanced cell lysis mediated by CD3xHer2 bispecific ligands. (d) PBMCs were co-cultured with SKBR3 at a fixed E:T = 10 in the presence of different concentrations of CD3xHer2, showing further enhancement of cell lysis with increasing ligand concentration. (e) PrA-PEG-SA molecular adaptor alone showed a lack of cytotoxicity in a concentration range up to 90 nM.
