Abstract
A tetrawalled and an octawalled molecular umbrella conjugate of amphotericin B (AmB) have been synthesized. Both conjugates exhibit high water solubility, a low tendency to aggregate, negligible hemolytic activity at 100 μM, and an ability to release a derivative of AmB under reducing conditions that exhibits high antifungal activity. Whereas the larger, octawalled conjugate shows slight adsorption to liposomal membranes and an ability to cross them by passive transport, the tetrawalled analogue shows significant adsorption and much lower bilayer transport activity. The potential of molecular umbrella–AmB conjugates as therapeutic agents is briefly discussed.
Short abstract
Please provide a synopsis
Amphotericin B (AmB) is an important antifungal agent that has been used to treat systemic fungal infections for more than half a century.1−4 Although considerable effort has been made to understand its mechanism of action, the means by which this macrolide antibiotic destroys fungal cells remain a matter of debate. In the most popular model, several AmB molecules are thought to combine with ergosterol to form water-filled pores (Figure 1).1 Subsequent thinning of the plasma membrane in the vicinity of this pore, or an alignment of two such pores across the membrane, is then presumed to result in the release of vital ions and ultimately cell death. Recently, evidence has been obtained indicating that AmB may also act by simply forming a complex with ergosterol.5
Figure 1.
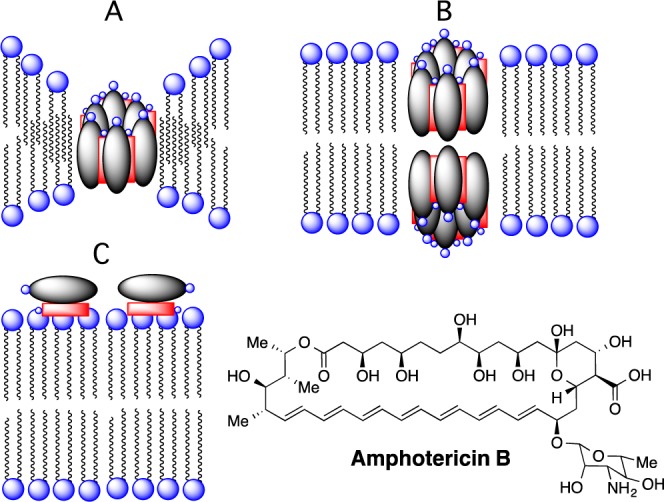
Barrel stavelike model in which AmB (gray oval) combines with ergosterol (red rectangle) to form single (A) or two aligned (B) water-filled pores or a simple complex (C).
Despite its efficacy in killing fungal cells, the use of AmB for treating fungal infections in the central nervous system (CNS) has proven challenging.6 In particular, the ability of AmB to penetrate the blood–brain barrier is known to be poor; i.e., concentrations of AmB in the CNS are barely measurable at the ng/mL level.7−9 To the best of our knowledge, no derivative of AmB has yet been reported that has exceeded the ability of AmB itself to cross the blood–brain-barrier; i.e., underivatized AmB remains as the drug of choice for clinical use. In principle, any improvement in transporting AmB across the blood–brain barrier could lead to a significant advance in treating fungal infections in the CNS. With this ultimate goal in mind, we sought to explore molecular umbrella–AmB conjugates as potential therapeutic alternatives.10
The primary aim of the present investigation was to test the hypothesis that molecular umbrella–AmB conjugates would (i) exhibit relatively high critical micelle concentrations, (ii) be nonhemolytic in their monomeric form, (iii) cross lipid bilayers by passive transport, and (iv) be capable of releasing a derivative of AmB having high antifungal activity. In principle, the generation of a high concentration gradient of such conjugates across the blood–brain barrier should facilitate the delivery of this AmB derivative into the CNS by increasing the driving force (i.e., chemical potential) for transport.
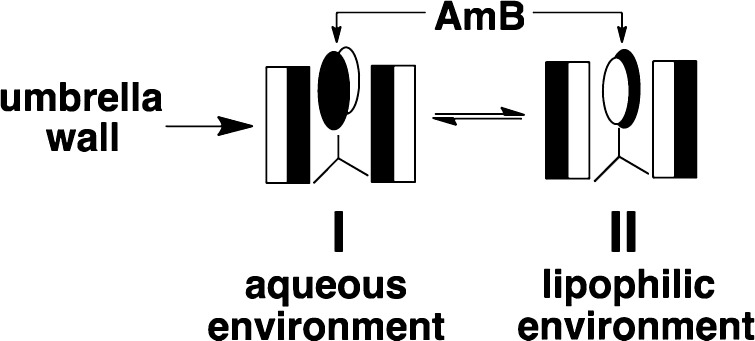
In brief, molecular umbrellas are molecules that contain two or more facial amphiphiles (i.e., “walls”) that are attached to a central scaffold.10 When immersed in a hydrophilic environment, these molecules have the potential for creating a hydrophilic exterior. Conversely, when immersed in a lipophilic environment, these same molecules have the potential for producing a lipophilic exterior. Since AmB is also facially amphiphilic, it too can act as wall. A stylized illustration of AmB that has been incorporated into a molecular umbrella is shown in Figure 2. In this illustration, the thermodynamically favored conformations that are expected in aqueous (I) and lipophilic (II) environments are depicted.
Figure 2.
Stylized illustration of a molecular umbrella–AmB conjugate favoring conformations in which the lipophilic faces of the umbrella and AmB are shielded (I) in an aqueous environment and exposed (II) in a lipophilic environment (II). The filled and open rectangles represent lipophilic and hydrophilic faces of each umbrella wall, respectively. The filled and open ovals represent the lipophilic and hydrophilic face of AmB, respectively.
Previously, we and others have shown that the hemolytic action of AmB requires that it attack red blood cells in an aggregated form.11,12 We have also shown that the attack of a simple detergent (Triton X-100) on cholesterol-rich liposomal membranes can lead to a “catastrophic rupture” of the bilayer but only when the detergent is in an aggregated state.13 Since molecular umbrella–AmB conjugates may shield their lipophilicity by adopting conformation I, their ability to aggregate in water, and also their hemolytic activity, is expected to be greatly reduced. In addition, their potential for undergoing conformational transitions from I to II and then back to I offers a plausible mechanism for diffusion from an aqueous environment across a lipid bilayer into an adjoining aqueous compartment (Figure 2). As discussed, elsewhere, this has been our working hypothesis.10
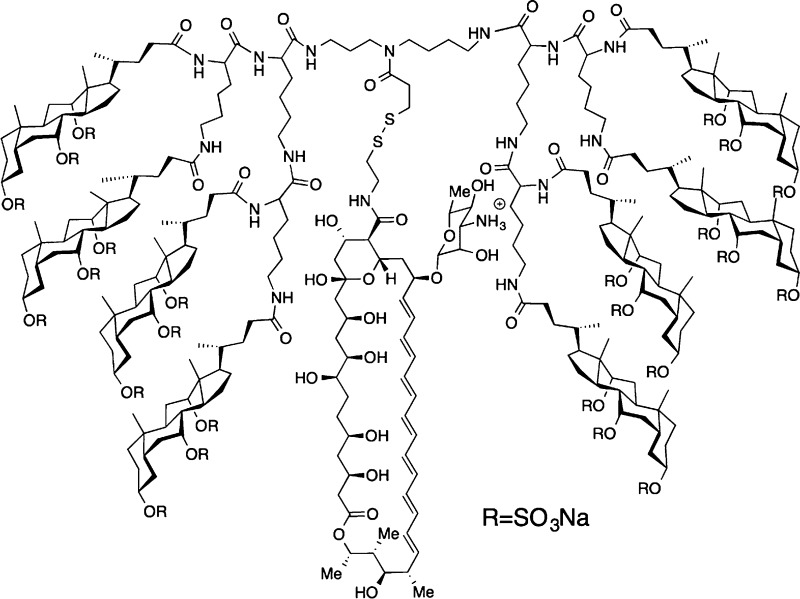
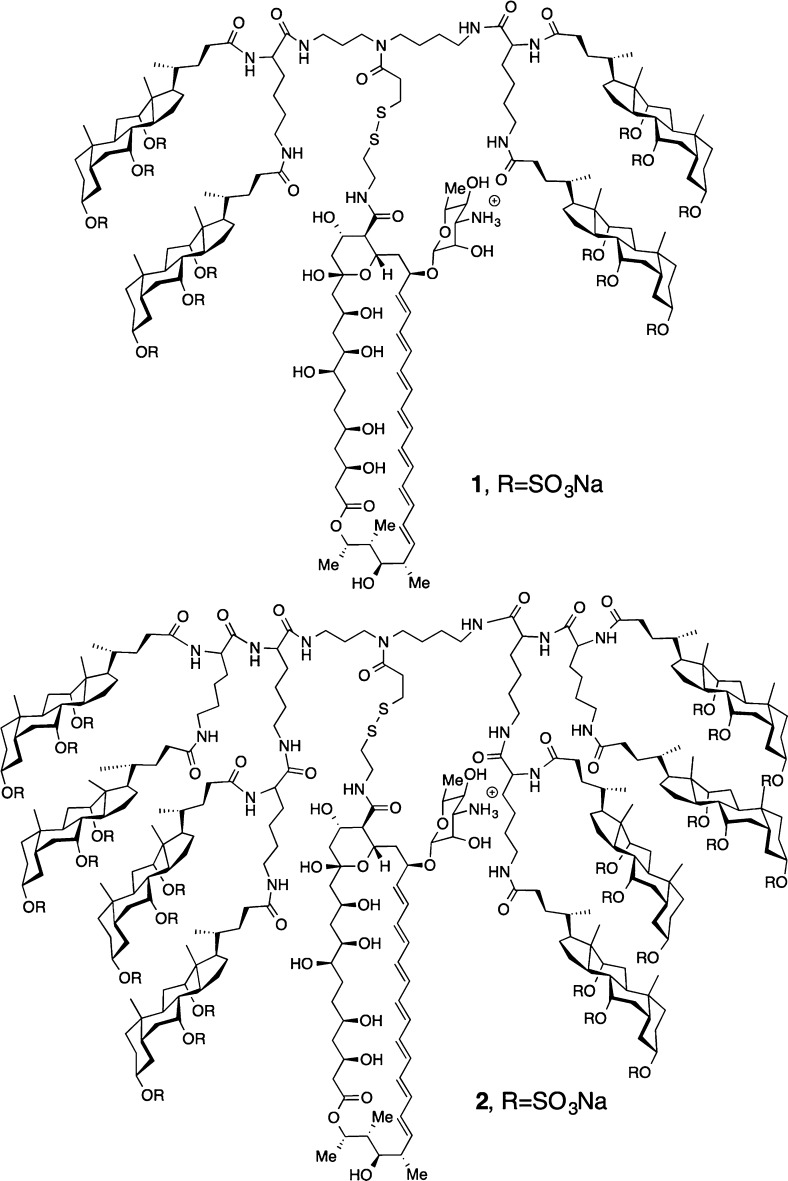
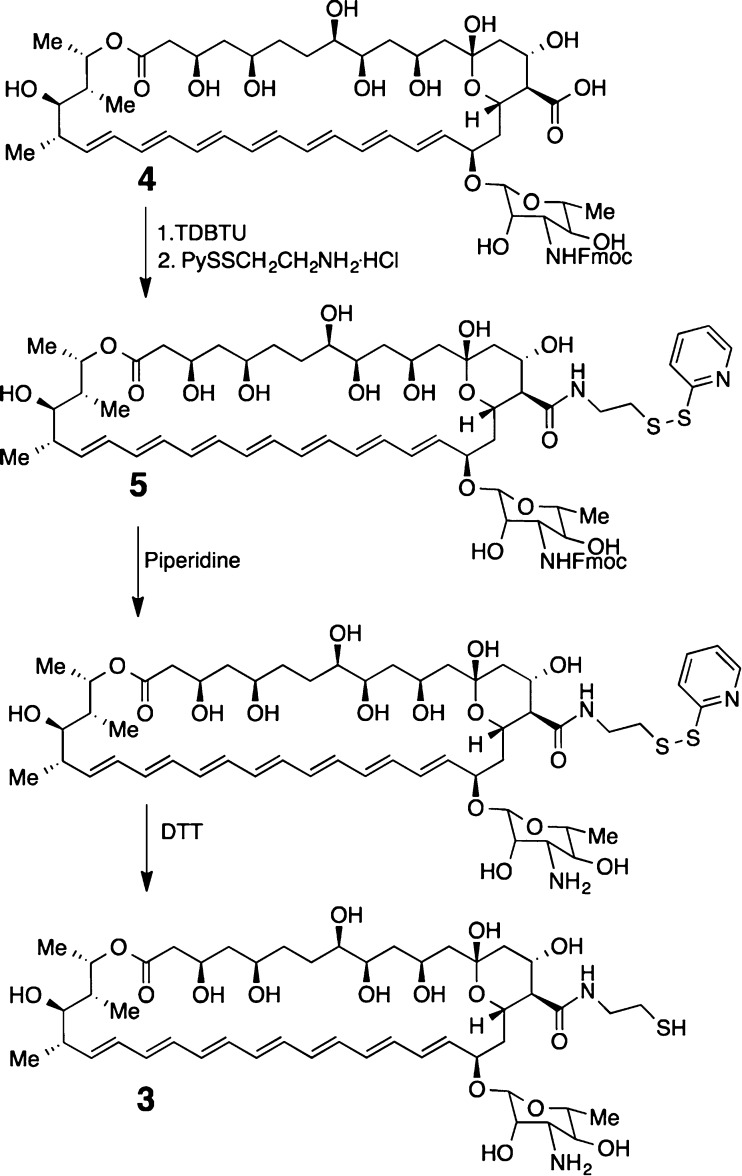
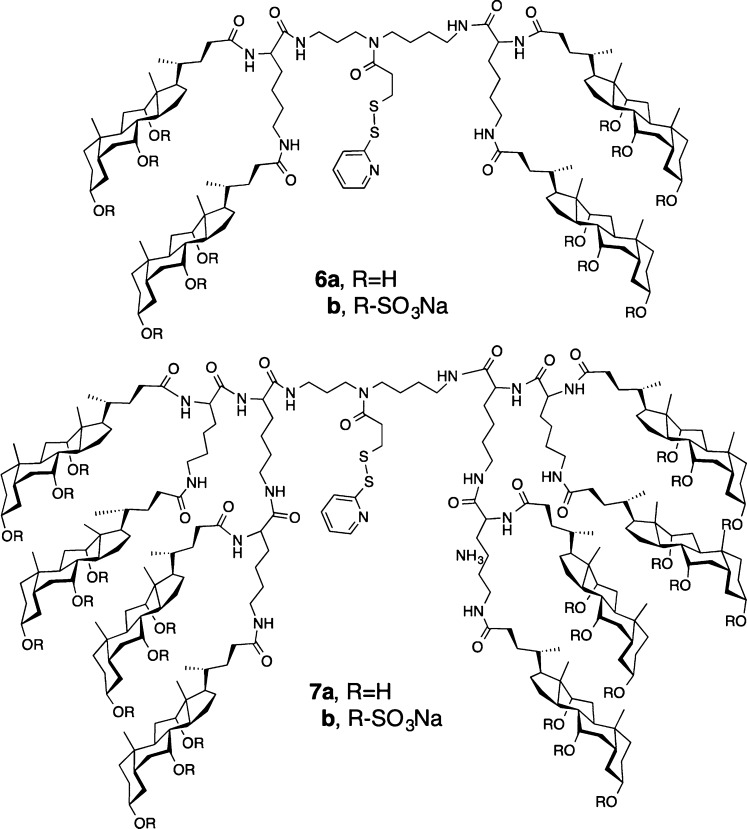
Two molecular umbrella–amphotericin B conjugates that were chosen as prototypes for the present investigation were the tetrawalled conjugate 1 and an octawalled analogue 2 (Chart 1). Tetrawalled and octawalled conjugates were selected based on our past success in using them to transport polar and nonpolar agents across lipid bilayers.10 A cleavable disulfide moiety was included in the molecular design to facilitate their synthesis and to confer prodrug properties. Thus, a thiol-bearing derivative of AmB, 3, was first synthesized as outlined in Scheme 1. Activation of previously reported Fmoc-protected AmB, 4, with N,N,N′,N′-tetramethyl-1,2-dihydrobenzo-1,2,3-triazineuronium tetrafluoroborate (TDBTU) and condensation with 2-pyridyldithioethylamine afforded 5.2 Subsequent deprotection with piperidine and dithiothreitol (DTT) then afforded 3. As expected, 3 exhibited strong antifungal properties. With 100% inhibition of growth as an end point, 3 showed the following activities against four different fungi: Candida albicans, 2 μg/mL; Candida glabrata, 2 μg/mL; Cryptococcus neoformans, 1 μg/mL; Cryptococcus gatti, 1 μg/mL. Under similar experimental conditions, AmB showed 100% inhibition of growth values that were 0.5, 0.5, 0.25, and 0.25 μg/mL, respectively. Preliminary experiments further indicate that 3, like AmB, is fungicidal.
Chart 1.
Scheme 1.
By use of procedures similar to those previously described, a tetrawalled and octawalled molecular umbrella precursor bearing a cleavable 2-thiopyridine moiety (i.e., 6a and 7a, respectively) were prepared (Chart 2).14,15 Both molecular umbrellas were then persulfated by treatment with sulfur trioxide pyridine complex to give 6b and 7b. Subsequent coupling of 3 via thiol–disulfide interchange in methanol afforded 1 and 2. Both of these molecular umbrella–AmB conjugates showed excellent solubility in water and a significant reduction in their tendency to aggregate relative to AmB. Also, 1 and 2 fully released 3 when subjected to reducing conditions, i.e., when subjected to excess dithiothreitol in methanol.
Chart 2.
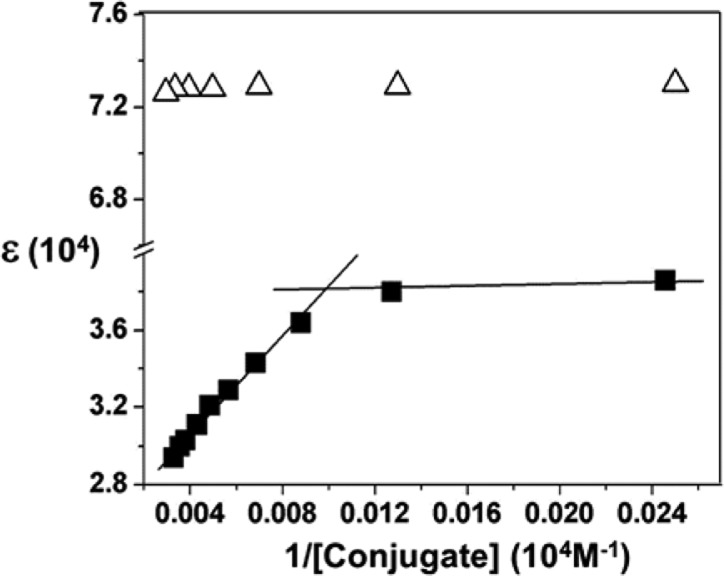
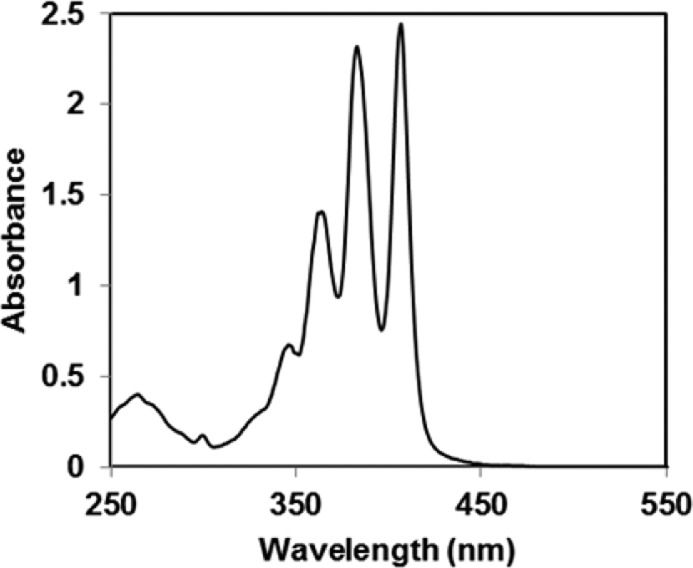
In its monomeric state, AmB exhibits a characteristic absorption at 409 nm with an apparent molar absorptivity that decreases upon aggregation.11 As discussed previously, if one lets (i) T, m, and P represent the total, the monomeric, and the micellar concentrations of this macrolide, respectively, (ii) ε represent the apparent molar absorptivity, and (iii) εm and εp represent the molar absorptivity for the monomeric and micellar components, respectively, then ε = εp + (εm – εp)m/T.11 At concentrations that are in excess of its cmc, m is constant and ε is expected to be inversely proportional to T. Thus, by measuring the apparent molar absorptivity as a function of the reciprocal of the macrolide concentration, one can estimate the cmc of the antibiotic from the intercept of two straight lines. Such plots are shown for 1 and 2 in Figure 3. While a cmc for 1 is evident at ∼100 μM, no aggregation was found for 2 at concentrations as high as 330 μM. To put these numbers into perspective, AmB exhibits a cmc of 1.1 μM under similar conditions.11 A UV spectrum that was recorded for 2 at 330 μM is shown in Figure 4.
Figure 3.
Plot of molar absorptivity (λmax = 409 nm) as a function of the reciprocal concentration of 1 (■) and 2 (△) in PBS at 37 °C.
Figure 4.
UV absorption spectrum of 2 (330 μM) in PBS at 37 °C.
As expected, 1 and 2 showed no hemolytic activity at relatively high concentrations. Thus, whereas 4 μM AmB was sufficient to produce 50% hemolysis of sheep red blood cells (4 × 107 cells/mL), no evidence of hemolysis was apparent for 1 and 2 at concentrations as high as 200 μM. In contrast to 3, which showed strong antifungal activity, both molecular umbrella–AmB conjugates showed no antifungal activity up to 100 μM against the same four fungi tested. The fact that these conjugates exhibit no antifungal activity can be accounted for by a shielding effect; i.e., the molecular umbrella covers the AmB moiety, which prevents the formation of barrel stave structures and ergosterol-based complexes (Figure 1).
To gain insight into the transport properties of 1 and 2, liposomes (200 nm, extrusion) were prepared from a mixture of 1-palmitoyl-2-oleyol-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG) [i.e., POPC/POPG, 95/5, mol/mol] in PBS containing a given concentration of 1 or 2. After an initial dialysis at 23 °C for 48 h to remove the conjugate from the external aqueous phase, the loss of the residual conjugate from the liposomes was then monitored at 37 °C (Figure 5).
Figure 5.
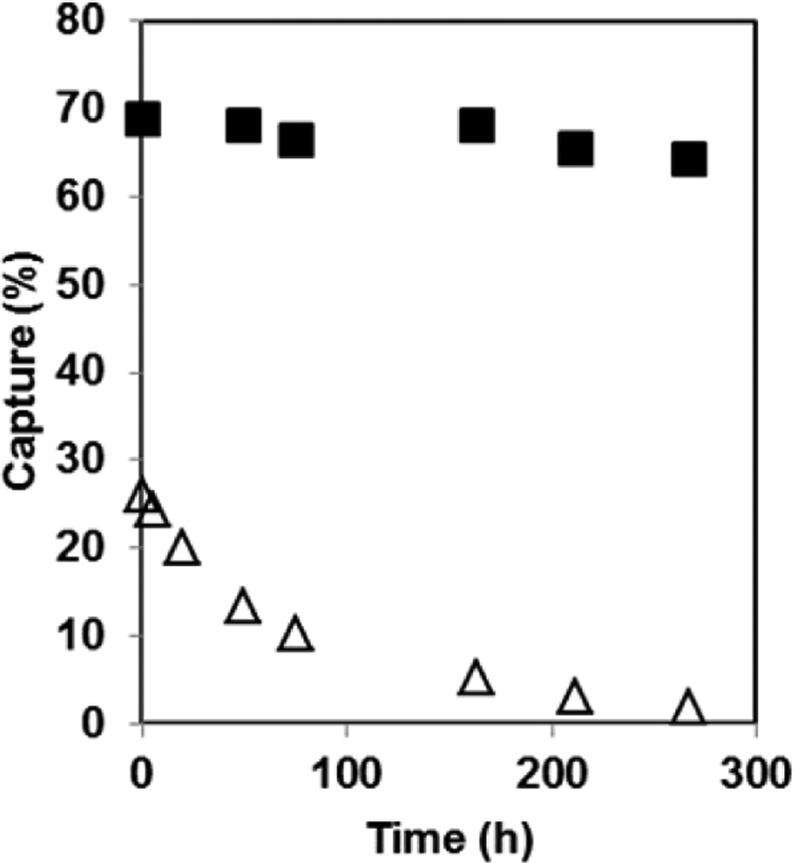
Plot of percent capture of 1 (■) and 2 (△) remaining with liposomes made from POPC/POPG (95/5, mol/mol) as a function of dialysis time at 37 °C after an initial dialysis period of 48 h at 23 °C.
On the basis of an average liposomal diameter of 200 nm (dynamic light scattering) and the concentration of lipid used (20 mg/mL), the theoretical percent capture of these molecular umbrella–AmB conjugates is ∼17%.16 This assumes that there is negligible absorption to the membrane. The percent capture refers to the percentage of the aqueous phase that is used in preparing the liposomes that becomes encapsulated within the aqueous interior of the liposomes. As shown in Figure 5, the apparent capture of 2 was slightly higher than this value, while that of 1 was much higher, reflecting modest and strong absorption to the liposomal membranes, respectively. Whereas the release of entrapped 2 was essentially complete after extended dialysis, the removal of entrapped and adsorbed 1 was close to negligible over the same time period. Examination of both liposomal dispersions before and after dialysis showed no change in their size or size distribution (dynamic light scattering). Incorporation of AmB in similar liposomal dispersions showed negligible release over the same time period of dialysis (not shown).
These results are similar to the transport behavior that we have reported previously for related tetrawalled and octawalled conjugates bearing a fluorescent dye (Cascade Blue); i.e., the larger persulfated molecular umbrellas crossed lipid bilayers more readily than smaller analogues.17 However, in sharp contrast, where the larger octawalled umbrella–dye conjugate showed higher membrane affinity and higher transport activity, the larger octawalled molecular umbrella–AmB conjugate (2) shows lower membrane affinity but higher transport activity. This finding further highlights the fact that the permeation across lipid bilayers is a multistep process consisting of adsorption, dehydration, diffusion, rehydration, and desorption and that depending on the nature of the molecule to be transported, as reflected by the number, type, and distribution of polar groups, its shape, and its hydrophilic/lipophilic balance, any one of these steps can become rate-limiting. The exact reason for this difference between these molecular umbrella-bound dye versus molecular umbrella-bound AmB conjugates is not presently clear.
The combination of high water solubility, a low tendency to aggregate, negligible hemolytic activity at 100 μM, an ability to cross lipid bilayers by passive transport, and release a potent antifungal agent (3) under reducing conditions provides considerable incentive for exploring the in vivo properties of 2 and related molecular umbrella–AmB conjugates in detail. Such studies are currently under active investigation.
Acknowledgments
This work was funded by the National Institutes of Health (Grant PHS GM100962).
Supporting Information Available
Experimental procedures used for chemical synthesis and physical measurements. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Bolard J. (1986) How do polyene macrolide antibiotics affect the cellular membrane properties?. Biochim. Biophys. Acta 864, 257–304. [DOI] [PubMed] [Google Scholar]
- Palacios D. S.; Anderson T. M.; Burke M. D. (2007) A post-PKS oxidation of the amphotericin B skeleton predicted to be crictical for channel formation is not required for potent antifungal activity. J. Am. Chem. Soc. 129, 13804–13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk B. C.; Goffeau A. (2008) Outwitting multidrug resistance to antifungals. Science 321, 367–369. [DOI] [PubMed] [Google Scholar]
- Volmer A. A.; Szpilman A. M.; Carreira E. M. (2010) Synthesis and biological evaluation of amphotericin B derivatives. Nat. Prod. Rep. 27, 1329–1349. [DOI] [PubMed] [Google Scholar]
- Gray K. C.; Palacios D. S.; Dailey I.; Endo M. M.; Uno B. E.; Wilcock B. C.; Burke M. D. (2012) Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U.S.A. 109, 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H., and Segal M. B. (1995) Physiology of the CSF and Blood-Brain Barriers, CRC Press, Boca Raton, FL. [Google Scholar]
- Perfect J. R.; Durack D. T. (1985) Comparison of amphotericin B and N-d-ornithyl amphotericin B methyl ester (SCH28191) in experimental cryptococcal meningitis and candida endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 28, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao K.; Huang R.; Li J.; Han L.; Ye L.; Lou J.; Jiang C. (2010) Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Controlled Release 147, 118–126. [DOI] [PubMed] [Google Scholar]
- Pyrgos V.; Mickiene D.; Sein T.; Cotton M.; Fransesconi A.; Mizrahi I.; Donoghue M.; Bundrant N.; Kim S. Y.; Hardwick M.; Shoham S.; Walsh T. J. (2010) Effects of immunomodulatory and organism-associated molecules on the permeability of an in vitro blood-brain barrier model to amphotericin B and fluconazole. Antimicrob. Agents Chemother. 54, 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janout V.; Regen S. L. (2009) Bioconjugate-based molecular umbrellas. Bioconjugate Chem. 20, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K.; Janout V.; Bernard E. M.; Armstrong D.; Regen S. L. (1995) Micelle/monomer control over the membrane-disrupting properties of an amphiphilic antibiotic. J. Am. Chem. Soc. 117, 6249–6253. [Google Scholar]
- Legrand P.; Romero E. A.; Cohen B. E.; Bolard J. (1992) Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob. Agents Chemother. 36, 2518–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Regen S. L. (1993) Control over vesicle rupture and leakage by membrane packing and by the aggregation state of an attacking surfactant. J. Am. Chem. Soc. 115, 708–713. [Google Scholar]
- Cline L. L.; Janout V.; Fisher M.; Juliano R. L.; Regen S. L. (2011) A molecular umbrella approach to the intracellular delivery of siRNA. Bioconjugate Chem. 22, 2210–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janout V.; Cline L. L.; Feuston B. P.; Klein L.; O’Brien A.; Tucker T.; Yuan Y.; O’Neill-Davis L. A.; Peiffer R. L.; Nerurkar S. S.; Jadhav V.; Tellers D. M.; Regen S. L. (2014) Molecular umbrella conjugate for the ocular delivery of siRNA. Bioconjugate Chem. 25, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1990) Liposomes: A Practical Approach (New R. R. C., Ed.) p 25, Oxford University Press, Oxford, U.K. [Google Scholar]
- Mehiri M.; Chen W.-H.; Janout V.; Regen S. L. (2009) Molecular umbrella transport: exceptions to the classic size/lipophilicity rule. J. Am. Chem. Soc. 131, 1338–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.