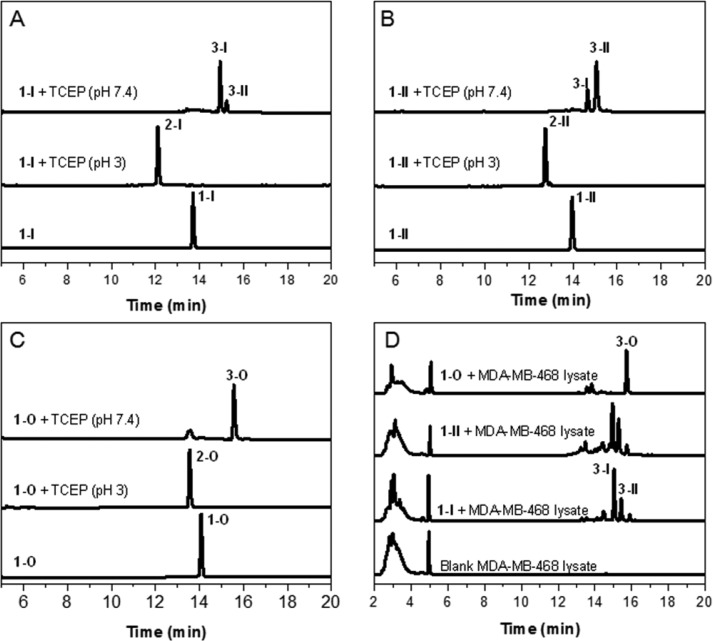
Figure 3.
Characterization of disulfide reduction-triggered macrocyclization in vitro. (A, B) HPLC traces of the incubation of 1-I and 1-II (20 μM) with reducing agent TCEP (200 μM) in aqueous solution. Bottom: 1-I (A) or 1-II (B) alone; middle: reduced intermediate 2-I (A) or 2-II (B) formed following 20 min TCEP reduction at pH 3; top: two cyclized diastereoisomeric products 3-I and 3-II formed following incubation of 1-I (A) or 1-II (B) with TCEP at pH 7.4 for 8 h. (C) HPLC traces of the incubation of 1-O (20 μM) with TCEP (200 μM) in aqueous solution. Bottom: 1-O alone; middle: reduced intermediate 2-O formed following 20 min TCEP reduction at pH 3; top: only one cyclized product 3-O formed following incubation of 1-O with TCEP at pH 7.4 overnight. (D) HPLC traces of the incubation solutions of 1-I, 1-II, and 1-O in cell lysates. Bottom to top: blank MDA-MB-468 cell lysates; 1-I, 1-II, or 1-O (100 μM) incubated in the MDA-MB-468 cell lysates overnight, respectively.