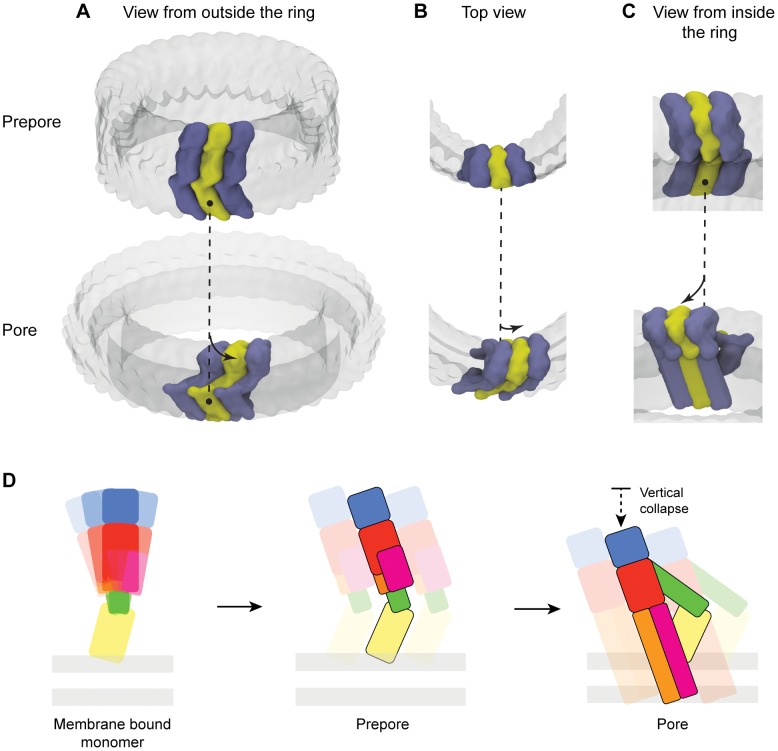
Figure 9. Orchestrated domain movement and proposed mechanism of pore formation.
Schematic representation of the proposed new model of prepore (top row) to pore (bottom row) transition shown from three point of views: A) from outside the ring formed by the oligomeric complex, B) Top view and C) from inside the ring. The dashed line symbolizes the position of Domain 4 in the prepore complex. The arrows symbolize the concerted movement experienced by the globular head domain (Domains 1 & 3) upon membrane insertion. Alternate monomers are represented with alternate colors; only three monomers are displayed for clarity; remaining monomers are represented as a transparent surface. D. Schematic representation of proposed CDC pore formation. The monomer displays flexibility in the orientation of Domains 1–3 versus the membrane binding Domain 4. Upon self-oligomerization into the prepore complex the monomer is trapped in a monomer accessible conformation. Upon membrane insertion, the orientation of Domain 2 flattens with respect to the membrane surface. This is accompanied by a vertical collapse of Domain 1 and 3, which brings them closer to the bilayer surface and allows insertion of TMH1/2 as β-hairpins. Colours are identical to Figure 1.