Abstract
Cancer is one of the most deadly diseases worldwide. In the last three decades many efforts have been made focused on understanding how cancer grows and responds to drugs. The dominant drug-development paradigm has been the “one drug, one target.” Based on that, the two main targeted therapies developed to combat cancer include the use of tyrosine kinase inhibitors and monoclonal antibodies. Development of drug resistance and side effects represent the major limiting factors for their use in cancer treatment. Nowadays, a new paradigm for cancer drug discovery is emerging wherein multi-targeted approaches gain ground in cancer therapy. Therefore, to overcome resistance to therapy, it is clear that a new generation of drugs is urgently needed. Here, regarding the concept of multi-targeted therapy, we discuss the challenges of using bacterial proteins and peptides as a new generation of effective anti-cancer drugs.
Keywords: cancer, monoclonal antibodies, tyrosine kinase inhibitors, therapeutic peptide/proteins, bacterial proteins, azurin
Introduction
The primary treatment modality for cancer involves surgical resection of the tumor(s) followed by radiation and chemotherapy. In some cases where the cancer, particularly pancreatic cancer, has advanced to a stage that precludes surgery, chemotherapy remains the standard of care.1 There are two types of drugs that are normally used in chemotherapy, both types usually guided by rational or structure based drug design. The first type are the small molecule drugs that target a single or limited number of key steps in cancer progression pathways, thereby significantly slowing down their growth. An advantage of such drugs is that they can often be administered orally. A second group of drugs comprises human and/or mammalian (but humanized) proteins, often monoclonal antibodies.2 A typical example of a structure-guided small molecule drug that inhibits a tyrosine kinase BCR-ABL is imatinib (Gleevec, Novartis) that was approved by the US FDA for the treatment of chronic myelogenous leukemia (CML), a cancer of bone marrow white blood cells triggered by the loss of regulation of a proto-oncogene protein tyrosine kinase.3 As CML cells became increasingly resistant to imatinib, other inhibitors such as dasatinib, nilotinib or bosutinib were developed and were approved by the US FDA for CML therapy.3 The receptor tyrosine kinases (RTKs) are members of a group of transmembrane proteins with extracellular and intracellular domains. The ligand binding at the extracellular domain allows autophosphorylation of the tyrosine residues at the intracellular domain of such tyrosine kinases as epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), etc., leading to downstream signaling and enhancement of proliferation, differentiation, and cellular growth of the cancer cells.2 Indeed, several inhibitors of the RTKs have been developed and approved by the FDA to treat a number of cancers (Table 1).
Table 1. Small-molecule tyrosine kinase inhibitors and monoclonal antibodies available in the market to treat human cancers.
| Generic and/or Trade name |
Target | Cancer | FDA approval |
|---|---|---|---|
| Tirosine kinase inhibitors | |||
|
Imatinib Gleevec |
BCR-ABL | Chronic Myeloid Leukemia Gastrointestinal stromal tumors |
2001 |
|
Gefitinib Iressa |
EGFR | Non-small Cell Lung Cancer | 2003 |
|
Erlotinib Tarceva |
EGFR | Non-small Cell Lung Cancer | 2004 |
|
Sorafenib Nexavar |
VEGFR | Hepatocellular Carcinoma Renal Cell Carcinoma |
2005 |
|
Dasatinib Sprycel |
BCR-ABL | Chronic Myeloid Leukemia | 2006 |
|
Sunitinib Sutent |
VEGFR | Renal Cell Carcinoma | 2006 |
|
Nilotinib Tasigna |
BCR-ABL | Chronic Myeloid Leukemia | 2007 |
|
Lapatinib Tyverb |
HER2 | Breast Cancer | 2007 |
|
Pazopanib Votrient |
VEGFR | Renal Cell Carcinoma | 2009 |
|
Vandetanib Zactima |
VEGFR | Thyroid | 2011 |
|
Vemurafanib Zelboraf |
B-Raf | Melanoma | 2011 |
|
Crizotinib Xalkori |
ALK | Non-small Cell Lung Cancer | 2011 |
|
Bosutinib Bosulif |
Src/Abl | Chronic Myeloid Leukemia | 2012 |
| Monoclonal antibodies | |||
|
Rituximab Rituxan |
CD20 | Chronic lymphocytic leukemia | 1997 |
|
Trastuzumab Herceptin |
HER2 | Metastatic Breast Cancer | 1998 |
|
Bevacizumab Avastin |
VEGF | Metastatic Colorectal Cancer | 2004 |
|
Cetuximab Erbitux |
EGFR | Colorectal Cancer | 2004 |
|
Panitumumab Vectibix |
EGFR | Colorectal Carcinoma | 2006 |
| Ofatumumab | CD20 | Chronic lymphocytic leukemia | 2009 |
| Ipilimumab | CTLA-4 | Melanoma | 2011 |
|
Pertuzumab Perjeta |
HER2 | Metastatic Breast Cancer | 2012 |
Small Molecule Anticancer Drugs
While among protein kinases, tyrosine kinase has been a favored target, other kinases such as serine and/or threonine kinase, as exemplified by the mammalian target of rapamycin (mTOR) belonging to the family of phosphoinositide 3-kinase (PI3K)-related kinases, have also recently been explored for inhibitor development. Similar to mutations in tumor suppressor genes such as TP53, leading to approximately 50% of human cancers, the mTOR signaling pathway is known to undergo mutational alterations in approximately 50% of human cancers, demonstrating high levels of phosphorylated intermediates in various clinical solid tumor samples. This has in turn led to recent attempts for developing orally-administered small molecule inhibitors of the mTOR signaling network.4 The classical mTOR inhibitor, the macrolide rapamycin, has been minimally successful as a candidate drug for cancers such as renal cell carcinoma or endometrial cancer, lacking a broad range of activity.5 The more recently developed small molecule inhibitors of mTOR kinase target both mTOR and mTOR/PI3K pathways that can be orally administered and where phase I/II clinical trials are on-going against cancers such as breast, lymphoma, malignant glioma, and others, either singly or in combination with known anticancer drugs such as vincristine and doxorubicin.6 The outcome of the clinical trials of these mTOR-specific kinase inhibitors is ongoing and their success will depend on the ability of the cancer cells to develop resistance as is common with the emergence of rapamycin-resistant mTOR signaling functions, as well as associated toxicities, particularly immunosuppression.6
Small molecule inhibitors are, however, not the only drugs targeted to cancer cell growth inhibition through limited target attack. Mammalian proteins, such as monoclonal antibodies (mAbs), have also been developed against selected target kinases and seven such mAbs are currently used in cancer therapy. A typical example will be Bevacizumab (Avastin, Table 1) which is a 149 kDa humanized IgG1 monoclonal antibody that selectively competes with vascular endothelial growth factor (VEGF), a 45 kDa glycoprotein. VEGF binds its receptor tyrosine kinase VEGFR, thereby initiating the signaling process and stimulating the growth of blood vessels, a process known as angiogenesis. Bevacizumab was approved as an antiangiogenic agent for the treatment of metastatic colorectal cancers in 2004 and is also used today for the treatment of glioblastomas. Similarly, trastuzumab has been shown to be effective for the treatment of patients with metastatic and early stage breast cancers that are positive for HER2.7 Many others such as cetuximab and panitumumab for EGFR positive, wild type KRAS-positive colorectal cancer, catumaxomab for EpCam positive malignant ascites, and rituximab for CD20-positive non-Hodgkin lymphoma, are currently in use (Table 1).8
There are two major problems with single (or limited) targeting drugs, even when the drug targets multiple members of the same family such as tyrosine kinases. Examples will be imatinib, sorafenib, and sunitinib which block several kinases, and therefore exhibit a range of activities against multiple cancers, as opposed to a single or limited number, targeting drug such as erlotinib or gefitinib. Because the target for these kinase inhibitors is often the ATP-binding pocket, the cancer cells can quickly change the target or switch to a different one, thereby developing resistance.9 For small molecule drugs, an active efflux system also plays a major role.9 There is also intrinsic resistance developed by cancer cells, as shown for tamoxifen, an anti-estrogenic agent, widely used for the treatment of breast cancer. To identify the nature of resistance development, comparative proteome analyses of over 5000 pooled tumor cells led to the identification of a protein EMMPRIN that appears to contribute to the resistance and may thus be used as a biomarker for the identification of tamoxifen resistance in recurrent breast cancer.10 The appearance of such resistance has led to the development of a new generation of kinase inhibitors, once the cancer becomes resistant to the first generation drug such as imatinib.9
A second major problem of the receptor tyrosine kinase inhibitors is their toxicity.2 As pointed out earlier,2 there are more than 500 kinases encoded by the human genome, and a critical evaluation of the cross-reactivity of a drug has shown that most of these drugs can block the activities of more than the kinase against which the inhibitor was selected, as exemplified by sorafenib, dasatinib, and sunitinib.11,12 Even the so-called single (or limited number) kinase targeting drug such as erlotinib or gefitinib targeting the EGFR and active against NSCLC or pancreatic cancer demonstrates significant side effects such as skin rash, diarrhea, nausea, and interstitial lung disease.2 Multi-kinase-targeting drugs such as sunitinib have been reported not only to demonstrate symptoms such as fatigue, nausea, anorexia, and skin rash but also neutropenia, lymphopenia, and anemia,12 as well as congestive heart failure, hypertension, and myocyte hypertrophy in patients with imatinib-resistant, metastatic gastrointestinal stromal tumors.13 It is important to note that the small molecule tyrosine kinase inhibitors are not the only ones to demonstrate cardiotoxicity or other toxicity symptoms. Hypertension, proteinuria, neutropenia, skin rash, etc., are common safety and health problems associated with mAbs such as bevacizumab, cetuximab, trastuzumab, etc., as well.2,14
The dual problems of quick resistance development and significant toxicity and side effects, while encouraging new drug development to combat resistance, have also encouraged development of medium to high molecular weight drugs, such as peptides and proteins, with larger number of targets and with a less stringent mode of action.9 The goal is not just to strongly inhibit a single type of pathway such as cell signaling mediated by the tyrosine kinases, but other pathways as well with less stringent mode of inhibition, so as not to elicit a quick and strong response from the cancer cells for resistance development.9 Modest inhibition of multiple pathways in the growth progression of cancer cells, while still contributing to significant growth inhibition, may elicit less toxicity-related problems, thereby addressing the dual problem of drug resistance and toxicity.9
Peptides as Potential Anticancer Drugs
Between small molecule compounds and high molecular weight protein molecules is a group of intermediate size compounds that comprise peptides that are often fragments of larger protein molecules. Peptides could comprise of just a few amino acids to about 40 or more amino acids coupled through amide and/or disulfide bonds, providing varied-size molecules. The number of peptide therapeutics that have entered the global market is about 10 with a few whose global sales exceeded US$ 1.0 billion in recent years, viz., Copaxone, Lupron, Zoladex, and Sandostatin.15 Six peptides received regulatory approval in 2012: Lucinactant, Peginesatide, Pasireotide, Carfilzomib, Linaclotide, and Teduglutide, including one (Lixisenatide) in 2013.16 While most of these therapeutic peptides are indicated for various diseases such as diabetes, anemia, etc., only a few such as Carfilzomib, a protease inhibitor, are indicated for hematological cancers such as multiple myeloma16 (Table 2). Interestingly, peptides, depending on their size or nature, can be administered orally, through subcutaneous or intravenous injections or even by inhalation. Although few in number, there are indications that peptide therapy may have interesting potential in cancer therapy. For example, similar to small molecule inhibitors of tyrosine kinases targeting EGFR involved in proliferation and migration of cancer cells, a synthetic six amino acid peptide of the alphaC-beta4 loop region of EGFR has been shown to inhibit the dimerization and signaling activity of EGFR in the presence of its ligand.17 This short peptide, targeting EGFR’s ATP-binding cleft and its dimerization face, additionally promotes EGFR interaction with the heat shock protein Hsp90, thereby catalyzing EGFR degradation as well.17 Indeed, several peptides such as Cilengitide, Trebananib, NGR-hTNF, Tyroserleutide, etc., are currently undergoing phase III clinical trials in patients with glioblastoma, ovarian, mesothelioma, or liver cancers.16 Chimeric peptides comprising a cationic domain and an apolipoprotein E receptor binding sequence have also been shown to allow delivery of therapeutic enzymes to the brain for use in neurodegenerative diseases.18
Table 2. Therapeutic peptides available in the market.
| Generic and/or Trade name |
Indications | FDA approval |
|---|---|---|
|
Leuprorelin Lupron |
Prostate cancer Breast cancer |
1985 |
|
Octreotide Sandostatin |
Prostate cancer Breast cancer |
1988 |
|
Goserelin Zoladex |
Prostate cancer Breast cancer |
1989 |
|
Glatiramer Copaxone |
Multiple sclerosis | 1996 |
|
Lucinactant Surfaxin |
Acute respiratory failure | 2012 |
|
Peginesatide Omontys |
Anemia (chronic kidney disease) | 2012 |
|
Pasireotide Signifor |
Cushing's disease | 2012 |
|
Carfilzomib Kyprolis |
Multiple myeloma | 2012 |
|
Linaclotide Linzess |
Chronic idiopathic constipation | 2012 |
|
Teduglutide Gattex |
Short bowel syndrome | 2012 |
|
Lixisenatide Lyxumia |
Diabetes | 2013 |
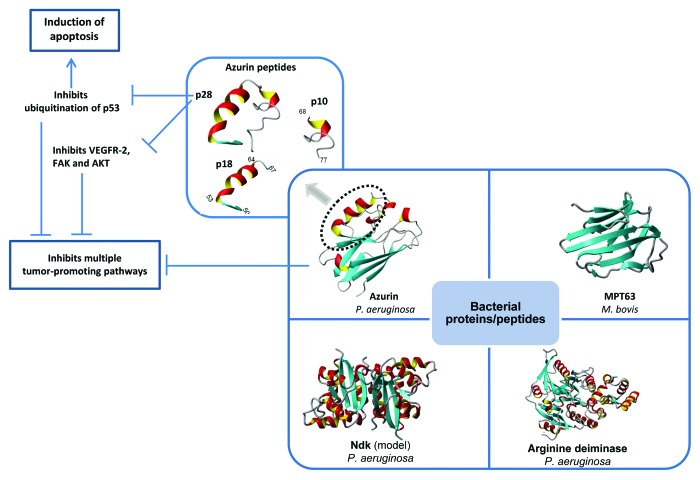
A major problem with the current approved therapeutic peptides is that none of them targets intracellular proteins, thus limiting their usefulness,15 particularly in cancer therapy. Although extracellular domains of receptor tyrosine kinases or similar signaling molecules promote cancer growth, many of the key components such as tumor suppressors or signaling proteins involved in cancer growth regulation are intracellular. Thus one key need for peptide therapeutic development for cancer therapy is the development of cell-penetrating peptides (CPP) that can cross the cellular membrane to modulate key intracellular proteins involved in cancer growth regulation. Well-known CP peptides have mostly been derived from heparin-, RNA- or DNA-binding proteins, antimicrobial or viral proteins as well as various natural proteins, a few of which are currently in clinical trial.15 Two such peptides, a helical peptide with a stretch of hydrophobic amino acids, termed p18, and an extended form of p18 with 10 additional amino acids, termed p28, have the unique capacity to enter preferentially to cancer cells but not the corresponding normal cells.19 P28 is a part of the bacterial protein azurin which not only enters preferentially to cancer cells but demonstrates strong anticancer activity as well (Fig. 1).2 Once internalized in cancer cells, p18 (azurin amino acids 50–67) has the protein transduction domain (PTD) but very little anticancer activity. Thus fluorescently-labeled p18 can be a good diagnostic marker to locate tumors inside the body where it accumulates because of selected entry (other than the kidney/liver). P28 (azurin 50–77), on the other hand, has not only preferential entry to the cancer cells but it forms a complex within the p53 DNA binding domain, inhibiting its ubiquitination and proteasomal degradation via an HDM-2 independent pathway.20 Such inhibition of p53 degradation raises the intracellular levels of this tumor suppressor, inducing apoptosis and cell cycle arrest in breast and other cancer cells.2,21
Figure 1. Bacterial proteins with anticancer properties. The anticancer activity of azurin, the primary focus of this article, resides, at least in part, within an extended 28-residues α helix termed p28. Full azurin as well as p28 peptide induce apoptosis and impair angiogenesis through multiple mechanisms. The phase I clinical study indicates that p28 peptide is safe and should be further considered as a promising anticancer therapeutic peptide.
Stabilization of p53 is not the only mode of action of the cell-penetrating peptide p28. In HUVEC cell model, p28 has been shown to inhibit angiogenesis in tumor cells by inhibiting phosphorylation of VEGFR-2, FAK, and AKT,22 and thus affecting tumor cell growth by inhibiting multiple independent pathways (Fig. 1).
Aside inhibition of tumor cell growth and induction of apoptosis in tumor cells because of its preferential entry and binding with intracellular p53 and angiogenesis-inducing proteins, p28 is also involved in preventing cancer induction in normal mouse ductal or alveolar mammary cells, when such cells were exposed to a potent carcinogen 7,12-dimethyl-benz-anthracene (DMBA). Treatment with DMBA allows induction of pre-cancerous lesions in the growing normal cells, and such lesion formation was shown to be inhibited by increasing concentrations of p28, as well as azurin, up to 70–75%, demonstrating both therapeutic and cancer preventive activity of p28.23-25
Will p28 work in cancer patients? This question was addressed by Richards et al.24 and Warso et al.26 in phase I clinical trials in Chicago with 15 stage IV cancer patients. When given as an intravenous bolus in 5 escalating doses in 15 stage IV cancer patients with refractory metastatic solid tumors (7 melanoma, 4 colon, 2 sarcoma, 1 pancreatic, and 1 prostate) 3 times per week over a 4 wk period with a two week break before the next, very little immunogenicity or toxicity was observed. Of the 15 patients with an average life expectancy of less than 6 mo, and who had solid tumors resistant to conventional drugs, 2 patients showed partial regression while 2 patients showed complete regression of their tumors at the end of the trial.24 Further follow up for another year and half after the termination of the trial demonstrated 3 patients with a partial response and one patient with a complete response for 139 wk.26 Three patients were reported alive at 158, 140, and 110 wk post therapy completion, demonstrating a clear anticancer activity without significant toxicity symptoms in stage IV cancer patients with drug-resistant tumors who had an average life expectancy of 26 wk or less.26
Bacterial Proteins as Potential Anticancer Drugs
Among well-known protein anticancer agents are immunotoxins,27 the mAbs mentioned in Table 1, and several bacterial proteins including Mycobacterium bovis MPT63,28 arginine deiminase from Mycoplasma arginini,29 lipidated azurin (Laz) from Neisseria meningitides,28 azurin from Pseudomonas aeruginosa,2 and others (Fig. 1). This review is primarily based on the P. aeruginosa protein azurin as a potential anticancer drug because of some of its unique properties.
Multivalent action of azurin toward cancer cells
As mentioned earlier, azurin, similar to p28 which is derived from azurin, can enter cancer cells much more preferentially than to normal cells.30 On entry into cancer cells, azurin interferes in cancer cell growth by multiple mechanisms including complex formation with the tumor suppressor protein p53,20 stabilizing it and enhancing its intracellular level, which then allows induction of apoptosis uniquely in cancer cells where it entered, leading to tumor cell death and shrinkage in mice.31 Similar to p28, azurin inhibits angiogenesis in cancer cells through inhibition of the phosphorylation of VEGFR-2, FAK, and AKT.22 But azurin has other cancer growth inhibitory activities that p28 lacks. For example, azurin does not have to go inside the cancer cells to form complexes with p53, VEGFR, FAK, AKT, and other cancer growth promoting proteins to inhibit their functions. There are many cancers that grow rapidly by hyperexpressing certain cell signaling receptor tyrosine kinase molecules on the cell surface and azurin can target these extracellular molecules. An example will be a receptor kinase EphB2 that is hyper-produced at the surface of many cancer cells such as breast, prostate, lung, etc., promoting their rapid growth and proliferation when bound with its cell-membrane associated ligand ephrin B2. It is important to note that azurin has interesting structural features that allow it to preferentially enter cancer cells and form complexes with key proteins involved in cancer growth to prevent their cancer growth promoting activity. In addition to the extended α-helix protein transduction domain (azurin 50–77) in the p28 region, azurin has in its C-terminal four loop regions termed CD loop, EF loop, FG loop, and GH loop as well as its structural similarity with antibody variable domains of various immunoglobulins giving rise to a β-sandwich core and an immunoglobulin fold. This allows azurin to evade immune action and exert its anticancer action when present in the blood stream, as shown in melanoma and breast tumor shrinkage studies in mice.32 Azurin has also been used in Escherichia coli Nissle 1917 cells through its hyper-expression in such cells to allow melanoma and breast tumor regression.33 Similarly, azurin’s binding domain to EphB2 via its G-H loop region (azurin 88–113) has been used to enhance radiation sensitivity of lung tumor cells through conjugation with the radio-sensitizer nicotinamide,34 two clever approaches utilizing azurin’s ability to attack a variety of cancers, including enhancing drug sensitivity to oral squamous carcinoma cells35 and others such as human osteosarcoma.36 As mentioned previously, azurin has other domains besides p28 such as p27 (azurin 88–113), where the chemically-synthesized p27 peptide had significant cytotoxic activity against EphB2-expressing prostate cancer,37 demonstrating the multi-domain and multivalent action of azurin to preferentially enter cancer cells and interfere in multiple steps in cancer growth, both intracellular and extracellular.
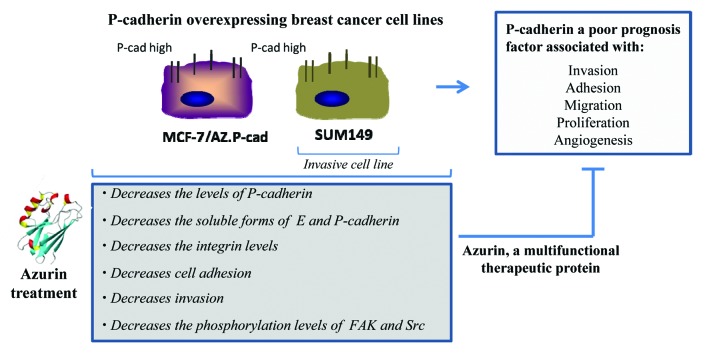
One of the more recent observations regarding the multivalent action of azurin toward cancer cells is its ability to inhibit the growth of highly invasive P-cadherin overexpressing breast cancer cells.38 P-cadherin is a member of the type I cadherin family that in certain conditions acts not as a regular cell-cell adhesion molecule, but as a promoter for malignant breast tumor progression39,40 (Fig. 2).
Figure 2. Multivalent anticancer action of azurin on P-cadherin overexpressing breast cancer cell lines.38
A sub-lethal single dose of azurin (with cell viability of at least 80%) produced a decrease in the invasion of two P-cadherin expressing breast cancer cell models, the luminal MCF-7/AZ.Pcad and the triple negative basal-like SUM 149 PT through a Matrigel artificial matrix. In both cell lines, the decrease in invasion was associated with a decrease in the total P-cadherin protein levels and a concomitant decrease of its membrane staining, whereas E-cadherin remains not altered with high expression levels and with normal membrane localization.38 The fact that in these models azurin interfered solely with P-cadherin protein expression but not E-cadherin, was a very important finding. Treating non-invasive cells, expressing E-cadherin (MCF-7/AZ.Mock), did not increase their invasion, revealing that azurin plays this important role only for the invasive cell lines.
This decrease in invasion and in P-cadherin levels was associated with other phenotypes associated to P-cadherin overexpression which were altered by azurin. The activity of MMP2, a metalloproteinase, in the extracellular media of cells was decreased.38 The proteolytic activity of MMP2 acts, in part, by shedding P-cadherin extracellular domain itself, releasing a soluble form of P-cadherin, sPcad12, which was also reduced in the extracellular media of azurin-treated cells. MMPs are involved in the degradation of the extracellular matrix, degrading several of its components, and particularly for MMP2, its active form has been detected in half of all human breast carcinomas.41 Adhesion molecules are also targets for matrix metalloproteinases. Also, for sPcad, its presence is associated with breast cancer patients: nipple aspirated fluids from breast cancer patients revealed increased shedding of this cell adhesion molecule than in healthy women or in pre-disease conditions.42 The fact that azurin has the ability to decrease the activity of these proteins may be of high clinical importance, but the exact mechanism remains elusive. However, it suggests that somehow, after azurin treatment, invasive cancer cells reduce the components that are more prone to the invasive function, among them P-cadherin, as a promoter for the models studied.
Azurin interferes with signaling pathways associated to cancer
Src and FAK are non-receptor tyrosine kinases very important for signaling cascades that mediate a number of biological processes associated with cell adhesion, both cell-to-cell and cell-to-ECM, migration and invasion.43-45 De-regulations in signaling from these two molecules are present in several cancer models, particular those with increased ability to migrate and invade into surrounding tissues. In accordance with previously published p28 peptide studies, it was also observed that in the same conditions where we observed the P-cadherin decrease and loss of invasive potential, p-FAK and p-Src were also decreased (Fig. 2).38 Src and FAK were identified as mediators of the crosstalk between integrin- and cadherin-mediated adhesion in epithelial cells and other cell surface receptor proteins.46,47
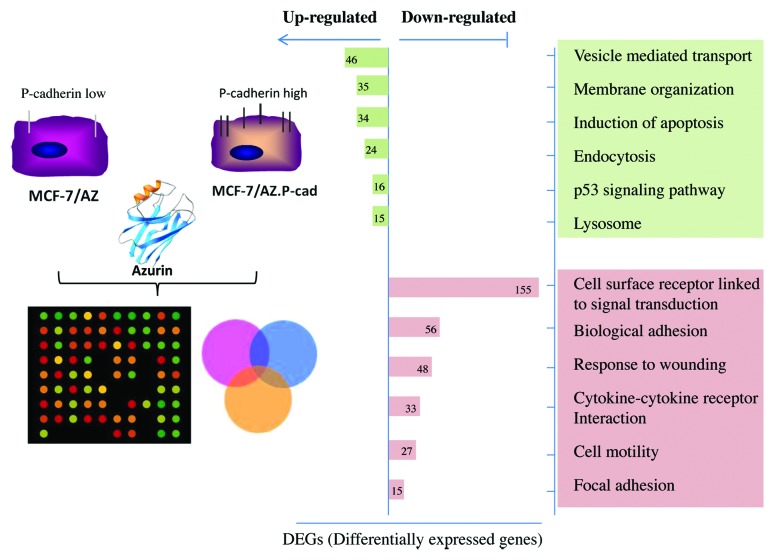
The alterations caused by azurin in biological processes associated with a more aggressive phenotype in invasive cancer cells were further studied in a transcriptomic profiling of invasive P-cadherin-overexpressing MCF-7/AZ.48 The majority of the genes downregulated were associated to adhesion of cells for their surroundings, either other cells or the Extracellular Matrix (ECM), and also with cell surface receptors and their consequent signal transduction partners (Fig. 3).
Figure 3. Array-based gene expression profile of azurin treated breast cancer cells.48 A genome-wide expression analysis of azurin-treated cells, reveals that azurin upregulated endocytic processes, concomitantly with the decrease in the expression of cell surface receptors and associated signaling, and decreased adhesion to the Extracellular Matrix (ECM).
On the other hand, azurin not only upregulated genes associated to apoptosis mediated by p53 protein, but also genes that were involved in endocytosis via vesicle-mediated transport and with lysosomal degradation, possibly of the cell surface receptors that became downregulated. Therefore, a possible mechanism by which azurin may act in certain conditions or cancer types is by decreasing membrane receptors and their hyperactivated signaling pathways that may be sustaining tumor proliferation. Other targets identified are integrin subunit receptors that bridge cells to the surrounding microenvironment, often hyper expressed in cancer cells and that help shape the ECM to favor tumor progression, such as integrin subunit β1.
Many of these receptors have been associated to tumor progression and resistance to standard therapies. Azurin, or the peptide, not only can act as a new therapeutic but also in combination with other agents, such as small molecule inhibitors of tyrosine kinases or monoclonal antibodies. This strategy could help improve the clinical efficacy of these agents and the reduction of tumor relapses in cancer patients.
Bacterial proteins in potential cancer therapy: Looking forward
Much discussion has recently centered on the need for multi-targeting drugs, whether analgesics or anticancer drugs, because of the complexity of neuronal systems or the complex network of signaling and growth promoting pathways that regulate cancer growth.49 As pointed out previously,9 single targeting, or drugs that target a few similar type of targets with strong inhibition, elicit quick response from the vulnerable cancer cells to resist such drug action. Another problem has been the process of drug development using the current rational or structure-based drug design where a drug competes selectively with a key molecule. For example, for tyrosine kinases, the target is the ATP-binding pocket where the intended drug competes with ATP for its binding to the pocket, thereby reducing ATP binding and the resultant kinase activity. Not only the cancer cells change or switch over the target binding pocket, but the drug can compete with ATP in binding tyrosine kinases involved in normal cell function, thus creating toxicity problems.9 It is thus interesting to note that within the limited number of weeks during which the clinical trials were conducted with p28, there was very little side effect seen while p28 allowed partial or sometimes complete regression of the drug-resistant tumors in 15 stage IV cancer patients.24,26 While selective entry in cancer cells is certainly one reason not to elicit toxic symptoms, the protein-protein complex formation seems to provide the specificity that is needed to reduce any toxic reaction. Whether such mode of cancer growth inhibition at multiple points will prevent, or reduce, drug resistance development can only be assessed after long-term use of azurin, if or when approved.
As discussed above, azurin has other domains in addition to its p28 domain that can make it a much more effective drug if its efficacy, lack of toxicity and hopefully a lack of susceptibility to resistance development can be demonstrated in pre-clinical and human clinical trials. A drawback of a protein drug, as is often illustrated with insulin, is its mode of administration, mostly through intravenous injections. Given azurin’s propensity for both therapeutic and cancer preventive activity, a weekly or bi-weekly injection of azurin in vulnerable people, for example women with family history of breast or ovarian cancers and with diagnosed BRCA1/BRCA2 mutations, may be one way to prevent, or greatly reduce, the onset of cancer in such people.25 There are, however, other approaches that are on the horizon for making protein drugs amenable to oral administration. Attempts are being made to chemically modify insulin with small polymers in which a single amphiphilic oligomer, often polyethylene glycol (PEG), is covalently linked to specific amino acids in insulin that allows it to resist degradation by the acids or enzymes in the stomach and intestine for absorption to the blood stream.50 Indeed, limited clinical trials using such conjugated, orally-administered insulin, for example oral insulin capsules (ORMD-0801, 8 mg insulin) have shown their tolerability and efficacy in reducing glycemia in eight type I diabetes patients.51 Since azurin (14 kDa) is also small, although somewhat larger than insulin (5.7 kDa), it should be possible to use such chemical modification to develop an oral variety of azurin (or p28 which is half the size of insulin) for treating and/or preventing the onset of cancer, once its lack of toxicity and anticancer efficacy is demonstrated in phase I/II clinical trials. Another exciting possibility, also on the horizon, is the use of bio-encapsulated proteins for oral delivery using plant cell expression.52,53 Using such emerging technology, azurin can be expressed in certain plant cells for oral consumption that would then allow azurin to be protected from stomach acids and proteolytic enzymes to be acted on by the intestinal microflora for passage through the intestinal lumen to the blood stream to reach the tumors. It is also noteworthy that all the four bacterial proteins, azurin, Laz, MPT63 and arginine deiminase, have anti-viral activity, including anti-HIV-AIDS activity.2 Thus if a bacterial protein such as azurin can be shown to be non-toxic in humans, it can be tested in both cancer and AIDS patients for its potential efficacy. Indeed, the role of bacterial proteins has been fictionalized in a book Bugging Cancer (http://logos-press.com/books/bugging-cancer/) to draw attention to the role microorganisms can play in our efforts to combat cancer and other diseases, as will also be discussed in a colloquium entitled Bugs as Drugs, organized by the American Academy of Microbiology in San Diego during April 9–11, 2014.
Finally, the development and marketing of an academic research-led potential anticancer protein drug such as azurin require not only hard work but a strong desire on the part of the university, and in fact the country, to translate university research to marketable commodities that help generate wealth both for the institution and the country. It’s thus no wonder that the University of Illinois applied for and owns more than 12 US patents and many international patents on azurin/p28 and issued an exclusive license to a start-up company CDG Therapeutics Inc. that in turn raised and invested more than 12 million dollars to conduct the research and phase I clinical trials with p28. Can similar efforts be reproduced in other universities in other countries? In a very meticulous, well-reasoned and thought-provoking article, Timmis et al.54 have argued for a strategy for the creation of pipelines for new chemicals, involving drugs and many other products, and encouraging national center network partnerships and stronger university-industry collaborations for wealth generation in cash-strapped Southern European countries. They also emphasize patent protection of new inventions, commercial assessment and stronger academic-industry partnership.54 Although the emphasis of the article of Timmis et al.54 is to encourage new chemical development, mostly natural products, in Southern European countries to promote an innovation-based industrial culture and economic revival, there are interesting differences among European Union (EU) countries in the legal enforcement of patent rights and in the conceptual framework of patent protection. As we pointed out earlier,55 while “anything under the sun that is made by man” is patent eligible in the United States (US Supreme Court decision 447 US 303, 1980), inventions that are contrary to public order or morality, including for example patenting of human embryonic stem cells, are not patent eligible under article 53 and rule 23 (c, d, e) of the European Patent Convention (EPC). While many innovations outlined by Timmis et al.54 will involve microorganisms which would be patent eligible in the EU, many involving higher forms of life will not. This then raises a broader issue, not between Northern and Southern Europe, but between the EU and the US about economic progress through patent protection of commercially valuable inventions, including for example, the evolving technology for using stem cells to generate human organs such as liver or lung for transplantation purposes. There is a need for frank and open discussions on how our society will benefit if we do not arbitrarily leave it to the industry or the governmental agencies to decide how our people, and our economy, can benefit from the academic innovations and a thoughtful strategy to bring such innovations to the bedside and the marketplace.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
N.B. acknowledges a Postdoctoral Fellowship from Fundação para a Ciência e a Tecnologia (FCT), Portugal. Research in the A.M. Fialho lab was supported by FCT (Grant PTDC/EBB/BIO/100326/2008).
References
- 1.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 2.Fialho AM, Chakrabarty AM. Promiscuous anticancer drugs from pathogenic bacteria: rational versus intelligent drug design. In Emerging Cancer Therapy Microbial Approaches and Biotechnological Tools. Fialho AM, Chakrabarty, AM, Eds. John Wiley, Hoboken, 2010. Pages 181-198. [Google Scholar]
- 3.Lambert GK, Duhme-Klair AK, Morgan T, Ramjee MK. The background, discovery and clinical development of BCR-ABL inhibitors. Drug Discov Today. 2013;18:992–1000. doi: 10.1016/j.drudis.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Don AS, Zheng XF. Recent clinical trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials. 2011;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YJ, Duan Y, Zheng XFS. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16:325–31. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artega CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER-2 positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson B, Bergh J. Hurdles in anticancer drug development from a regulatory perspective. Nat Rev Clin Oncol. 2012;9:236–43. doi: 10.1038/nrclinonc.2012.14. [DOI] [PubMed] [Google Scholar]
- 9.Avner BS, Fialho AM, Chakrabarty AM. Overcoming drug resistance in multi-drug resistant cancers and microorganisms: a conceptual framework. Bioengineered. 2012;3:262–70. doi: 10.4161/bioe.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umar A, Kang H, Timmermans AM, Look MP, Meijer-van Gelder ME, den Bakker MA, Jaitly N, Martens JW, Luider TM, Foekens JA, et al. Identification of a putative protein profile associated with tamoxifen therapy resistance in breast cancer. Mol Cell Proteomics. 2009;8:1278–94. doi: 10.1074/mcp.M800493-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 12.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 13.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 15.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–60. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today. 2013;18:807–17. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Ahsan A, Ray D, Ramanand SG, Hegde A, Whitehead C, Rehemtulla A, Morishima Y, Pratt WB, Osawa Y, Lawrence TS, et al. Destabilization of the epidermal growth factor receptor (EGFR) by a peptide that inhibits EGFR binding to heat shock protein 90 and receptor dimerization. J Biol Chem. 2013;288:26879–86. doi: 10.1074/jbc.M113.492280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Y, Sohar I, Sleat DE, Richardson JR, Reuhl KR, Jenkins RB, Sarkar G, Lobel P. Effective intravenous therapy for neurodegenerative disease with a therapeutic enzyme and a peptide that mediates delivery to the brain. Mol Ther. 2014;22:547–53. doi: 10.1038/mt.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor BN, Mehta RR, Yamada T, Lekmine F, Christov K, Chakrabarty AM, Green A, Bratescu L, Shilkaitis A, Beattie CW, et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 2009;69:537–46. doi: 10.1158/0008-5472.CAN-08-2932. [DOI] [PubMed] [Google Scholar]
- 20.Bizzarri AR, Santini S, Coppari E, Bucciantini M, Di Agostino S, Yamada T, Beattie CW, Cannistraro S. Interaction of an anticancer peptide fragment of azurin with p53 and its isolated domains studied by atomic force spectroscopy. Int J Nanomedicine. 2011;6:3011–9. doi: 10.2147/IJN.S26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada T, Mehta RR, Lekmine F, Christov K, King ML, Majumdar D, Shilkaitis A, Green A, Bratescu L, Beattie CW, et al. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol Cancer Ther. 2009;8:2947–58. doi: 10.1158/1535-7163.MCT-09-0444. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RR, Yamada T, Taylor BN, Christov K, King ML, Majumdar D, Lekmine F, Tiruppathi C, Shilkaitis A, Bratescu L, et al. A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis. 2011;14:355–69. doi: 10.1007/s10456-011-9220-6. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RR, Hawthorne M, Peng X, Shilkaitis A, Mehta RG, Beattie CW, Das Gupta TK. A 28-amino-acid peptide fragment of the cupredoxin azurin prevents carcinogen-induced mouse mammary lesions. Cancer Prev Res (Phila) 2010;3:1351–60. doi: 10.1158/1940-6207.CAPR-10-0024. [DOI] [PubMed] [Google Scholar]
- 24.Richards JM, Warso MA, Mehta D, Christov K, Schaeffer C, Rae Bressler L, Yamada T, Majumdar D, Kennedy SA, Beattie CW, et al. A first-in-class, first-in-human phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with metastatic refractory solid tumors. J Clin Oncol 2011; 29: abstract 2511 (ASCO Annual Meeting). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fialho AM, Chakrabarty AM. Patent controversies and court cases: cancer diagnosis, therapy and prevention. Cancer Biol Ther. 2012;13:1229–34. doi: 10.4161/cbt.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warso MA, Richards JM, Mehta D, Christov K, Schaeffer C, Rae Bressler L, Yamada T, Majumdar D, Kennedy SA, Beattie CW, et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br J Cancer. 2013;108:1061–70. doi: 10.1038/bjc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhary S, Mathew M, Verma RS. Therapeutic potential of anticancer immunotoxins. Drug Discov Today. 2011;16:495–503. doi: 10.1016/j.drudis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Fialho AM, Salunkhe P, Manna S, Mahali S, Chakrabarty AM. Glioblastoma multiforme: novel therapeutic approaches. ISRN Neurol. 2012;2012:642345. doi: 10.5402/2012/642345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feun L, Tien Kuo M, You M, Wu CJ, Wangpaichitr M, Savaraj N. Arginine deiminase and cancer therapy. In Emerging Cancer Therapy: Microbial Approaches and Biotechnological Tools. Fialho AM, Chakrabarty AM, Eds. John Wiley, Hoboken, 2010. Pages 199-217. [Google Scholar]
- 30.Yamada T, Fialho AM, Punj V, Bratescu L, Das Gupta TK, Chakrabarty AM. Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol. 2005;7:1418–31. doi: 10.1111/j.1462-5822.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamada T, Hiraoka Y, Ikehata M, Kimbara K, Avner BS, Das Gupta TK, Chakrabarty AM. Apoptosis or growth arrest: Modulation of tumor suppressor p53’s specificity by bacterial redox protein azurin. Proc Natl Acad Sci U S A. 2004;101:4770–5. doi: 10.1073/pnas.0400899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fialho AM, Stevens FJ, Das Gupta TK, Chakrabarty AM. Beyond host-pathogen interactions: microbial defense strategy in the host environment. Curr Opin Biotechnol. 2007;18:279–86. doi: 10.1016/j.copbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang Y, Xia L, Zhang X, Ding X, Yan F, Wu F. Escherichia coli Nissle 1917 targets and restrains mouse B16 melanoma and 4T1 breast tumors through expression of azurin protein. Appl Environ Microbiol. 2012;78:7603–10. doi: 10.1128/AEM.01390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micewicz ED, Jung C-L, Schaue D, Luong H, McBride WH, Ruchala P. Small azurin derived peptide targets ephrin receptors for radiotherapy. Int J Pept Res Ther. 2011;17:247–57. doi: 10.1007/s10989-011-9265-9. [DOI] [Google Scholar]
- 35.Cho JH, Lee MH, Cho YJ, Park BS, Kim S, Kim GC. The bacterial protein azurin enhances sensitivity of oral squamous carcinoma cells to anticancer drugs. Yonsei Med J. 2011;52:773–8. doi: 10.3349/ymj.2011.52.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D-S, Miao XD, Ye ZM, Feng J, Xu RZ, Huang X, Ge FF. Bacterial redox protein azurin induce apoptosis in human osteosarcoma U2OS cells. Pharmacol Res. 2005;52:413–21. doi: 10.1016/j.phrs.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, Hashimoto W, Schlarb-Ridley B, Cho W, Das Gupta TK, et al. Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry. 2007;46:1799–810. doi: 10.1021/bi061661x. [DOI] [PubMed] [Google Scholar]
- 38.Bernardes N, Ribeiro AS, Abreu S, Mota B, Matos RG, Arraiano CM, Seruca R, Paredes J, Fialho AM. The bacterial protein azurin impairs invasion and FAK/Src signaling in P-cadherin-overexpressing breast cancer cell models. PLoS One. 2013;8:e69023. doi: 10.1371/journal.pone.0069023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paredes J, Albergaria A, Oliveira JT, Jerónimo C, Milanezi F, Schmitt FC. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res. 2005;11:5869–77. doi: 10.1158/1078-0432.CCR-05-0059. [DOI] [PubMed] [Google Scholar]
- 40.Albergaria A, Ribeiro AS, Vieira AF, Sousa B, Nobre AR, Seruca R, Schmitt F, Paredes J. P-cadherin role in normal breast development and cancer. Int J Dev Biol. 2011;55:811–22. doi: 10.1387/ijdb.113382aa. [DOI] [PubMed] [Google Scholar]
- 41.Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853–68. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannello F, Tonti GA, Medda V, Pederzoli A, Sauter ER. Increased shedding of soluble fragments of P-cadherin in nipple aspirate fluids from women with breast cancer. Cancer Sci. 2008;99:2160–9. doi: 10.1111/j.1349-7006.2008.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Luo M, Guan JL. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2010;289:127–39. doi: 10.1016/j.canlet.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–7. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J Cell Sci. 2012;125:497–506. doi: 10.1242/jcs.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernardes N, Ribeiro AS, Abreu S, Vieira AF, Carreto L, Santos M, Seruca R, Paredes J, Fialho AM. High-throughput molecular profiling of a P-cadherin overexpressing breast cancer model reveals new targets for the anti-cancer bacterial protein azurin. Int J Biochem Cell Biol. 2014;50:1–9. doi: 10.1016/j.biocel.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Ohlson S. Designing transient binding drugs: a new concept for drug discovery. Drug Discov Today. 2008;13:433–9. doi: 10.1016/j.drudis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Shaji J, Patole V. Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci. 2008;70:269–77. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eldor R, Arbit E, Corcos A, Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8:e59524. doi: 10.1371/journal.pone.0059524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SL, Li Q, Daniell H. Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol Ther. 2014;22:535–46. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimran A, Brill-Almon E, Chertkoff R, Petakov M, Blanco-Favela F, Muñoz ET, Solorio-Meza SE, Amato D, Duran G, Giona F, et al. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood. 2011;118:5767–73. doi: 10.1182/blood-2011-07-366955. [DOI] [PubMed] [Google Scholar]
- 54.Timmis K, de Lorenzo V, Verstraete W, Garcia JL, Ramos JL, Santos H, Economidis I, Nogales B, Timmis JK, Fonseca C, et al. Pipelines for New Chemicals: a strategy to create new value chains and stimulate innovation-based economic revival in Southern European countries. Environ Microbiol. 2014;16:9–18. doi: 10.1111/1462-2920.12337. [DOI] [PubMed] [Google Scholar]
- 55.Fialho AM, Chakrabarty AM. The role and importance of intellectual property generation and protection in drug development. In Emerging Cancer Therapy: Microbial Approaches and Biotechnological Tools. Fialho AM, Chakrabarty AM, Eds. John Wiley, Hoboken, 2010. Pages 405-419. [Google Scholar]