FIGURE 2.
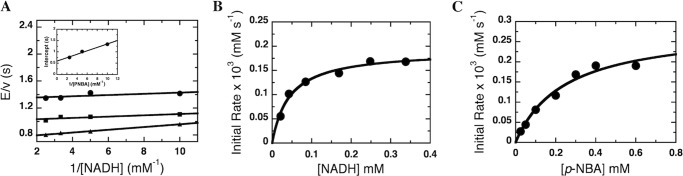
Steady-state kinetics of NR with NADH and p-NBA as substrates. A, double-reciprocal plots of the initial rates of reaction of NR with various concentrations of NADH (0.1, 0.2, 0.3, and 0.4 mm after mixing). Upper to lower lines of the primary plot are from the reactions of 0.1, 0.2, and 0.4 mm (after mixing) p-NBA, respectively. The assay reactions were performed using a stopped-flow spectrophotometer at 4 °C. The inset shows the plot of ordinate intercepts from the double-reciprocal plot versus the reciprocal of p-NBA concentrations. The parameters obtained from the fits are provided in Table 1. B and C, used an enzyme concentration of 118 nm versus 60 nm for panel A. B, plot of initial velocity versus NADH concentration (0.02, 0.042, 0.08, 0.17, 0.25, and 0.34 mm) at a fixed p-NBA concentration (0.4 mm). The results show hyperbolic dependence on NADH concentrations with KmNADH of 44 μm and kcat = 1.7 s−1. C, plot of initial velocity versus p-NBA concentration (0.025, 0.050, 0.1, 0.2, 0.3, 0.4, and 0.6 mm) at a fixed NADH concentration (0.25 mm). The results showed hyperbolic dependence on p-NBA concentrations with the KmPNBA of 0.25 mm and kcat = 1.7 s−1.
