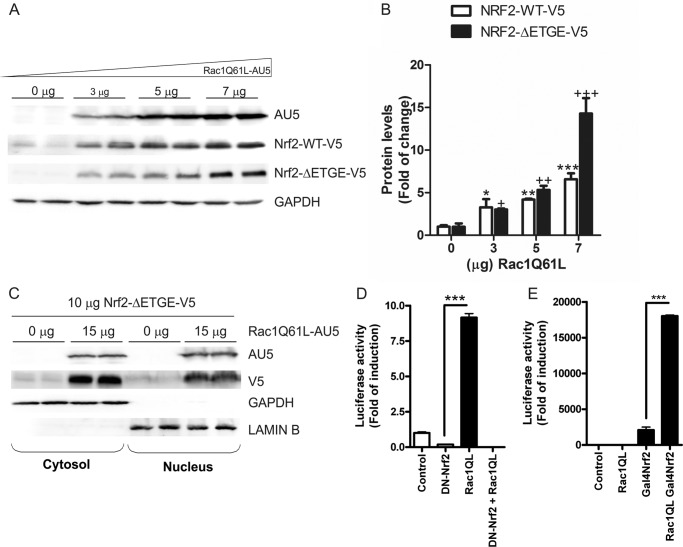
FIGURE 3.
RAC1 induces NRF2 signaling pathway. A, RAC1 induces NRF2 expression in a dose-dependent manner. Cells were transfected with different doses of RAC1Q61L and a constant amount of V5-tagged NRF2 or NRF2ΔETGE-mutant plasmids. Upper panels, immunoblots with anti-AU5 antibody; middle panel, immunoblots with anti-V5; lower panel, anti-GAPDH as protein load control. B, quantification of immunoblots for NRF2-WT-V5 and NRF2-ΔETGE-V5. One-way ANOVA followed by Newman-Keuls test was used to assess differences among groups as previously. C, RAC1 induces NRF2 nuclear translocation. Cells were co-transfected with or without RAC1Q61L and a constant dose of NRF2ΔETGE-V5 plasmid, and subcellular fractionation was performed. Upper panels, immunoblots with anti-AU5 or V5 antibodies; middle panel, immunoblots with GAPDH antibody as a control for cytosolic protein load; lower panel, anti-lamin B as a control for nuclear protein load. D, RAC1 requires NRF2 to activate the HO-1 promoter. HEK293T cells were co-transfected with RAC1Q61L expression vector, pHO1–15-LUC, Renilla control vectors, and either empty vector or dominant negative NRF2, (DN)-NRF2, expression vector. E, RAC1 uses the transactivating activity of NRF2 to induce AREs. Cells were co-transfected with Gal4-LUC (or pGL3-basic as a control) and either empty vector of expression vectors for Gal4NRF2 and RAC1 as indicated. Luciferase experiments were performed at least three times using three-four samples per group. The values in graphs correspond to the mean ± S.E. Student's t test was used to assess differences between groups or one-way ANOVA followed by a Newman-Keuls post-test (C). Asterisks denote statistically significant differences with: *, p < 0.05; **, p < 0.01; ***, p < 0.001.