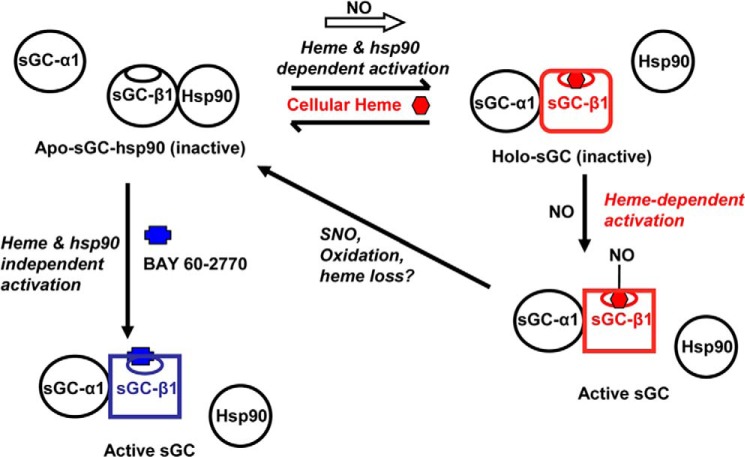
FIGURE 8.
Model that connects sGC-β1 protein interactions, heme content, and activity and shows the influence of heme-dependent (NO) or heme-independent (BAY 60-2770) sGC activators. An equilibrium exists in cells between a hsp90-bound apo-sGC-β1 (top left, black β subunit) and a heme-replete sGC-β1 that is instead associated with sGC-α1 (top right, red β subunit). NO can rapidly shift this equilibrium to the right when cell heme levels are sufficient and hsp90 is active. NO can then bind to the heme in the sGC heterodimer and activate catalysis (bottom right). The distinct structural changes in the sGC-β1 subunit caused by the heme insertion and NO binding steps are indicated by changes in the β subunit shape. Further NO exposure may cause S-nitrosation (SNO) of sGC-β1 and heme oxidation/loss and thereby desensitize sGC toward NO and promote its hsp90 reassociation. Binding of the heme-independent activator BAY 60-2770 (blue) to the apo-sGC-hsp90 species can occur independently of active hsp90 and cellular heme, and this triggers the same changes in sGC-β1 structure and protein interactions that are needed to activate its catalysis (lower left, blue β subunit).