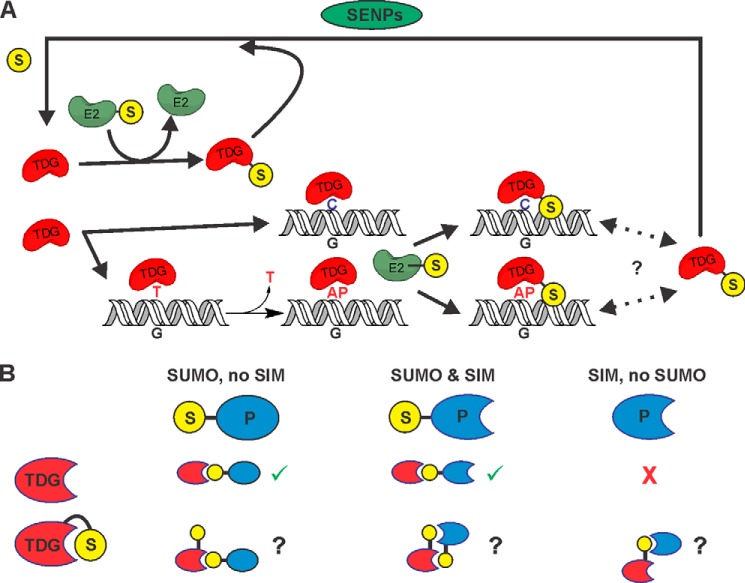
FIGURE 8.
Summary of findings from these and previous studies regarding TDG sumoylation. A, our results showed that E2∼SUMO efficiently modifies free and DNA-bound TDG, exhibiting no substantial specificity for product-bound TDG. Thus, the previous proposal that TDG is selectively sumoylated when it is bound to AP-DNA (just after base excision) would require an unidentified E3 or other selectivity factor (16). Nevertheless, modification of product-bound TDG by E2∼SUMO is relatively efficient and could potentially stimulate dissociation of the product complex (in the absence of an E3), thereby enhancing enzymatic turnover. Given our finding that TDG∼SUMO-1 still binds AP-DNA with high affinity, it is possible that TDG∼SUMO-1 shuttles on and off of AP-DNA (or undamaged DNA), rather than dissociating completely (as indicated by dotted lines and question mark). However, because TDG∼SUMO lacks G·T activity and E2 modifies free and DNA-bound TDG, efficient desumoylation (by SENPs) is needed to maintain a pool of catalytically active TDG. B, sumoylation of free TDG can modulate its interactions with other proteins (P), which can depend on whether they contain a SIM or are themselves sumoylated. Crystallographic studies (17, 18) indicate that for sumoylated TDG, the covalently tethered SUMO domain occupies its own SIM, which could potentially suppress interactions with a sumoylated partner protein, unless it also contains a SIM. Potential SUMO-mediated interactions of free and modified TDG with sumoylated and/or SIM-containing partners are shown. Checkmarks (in green) indicate allowed SUMO/SIM interactions, question marks indicate interactions that would require a change in SUMO conformation to occur, and crosses (in red) indicate SUMO/SIM-mediated interactions that should not occur.