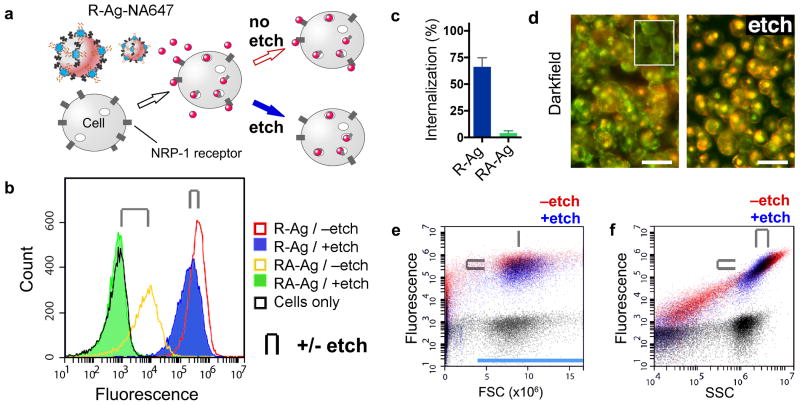
Figure 3. Flow cytometry with AgNPs.
(a) Scheme of CendR peptide RPARPAR dependent R-Ag-NA647 binding to NRP-1 expressing cells. After splitting into two samples one is etched. (b) Fluorescence histograms of cells with Ag-NA647 carrying either of two peptides. Cell-gated plots of internalizing R-Ag-NA647 (red, -etch), and with etch (blue), versus non-internalizing control, RPARPARA (RA)-Ag-NA647 (yellow, -etch) and with etch (green). Cells without Ag were included as a control (black). Paired +/- etch is indicated in all panels by the clip icon. (c) Internalization into cells was quantified from b as the percent of mean signal retained after etching. Error bars (S.D.) were generated across five separate incubations and cytometric runs. (d) Darkfield imaging of cell suspensions with R-Ag-NA647 shows that etched cells lose the membrane puncta (Ag) but retain the perinuclear (and red-shifted) scattering spectra from internalized Ag. Inset shows cells without AgNPs. Scale bars are 25 μm. (e) R-Ag-NA647 from b plotted as ungated dot plots in fluorescence versus forward scatter (FSC) or (panel f) side scatter (SSC). Black dot plots are from cells-only control. In e, FSC detected cells but the signal did not shift when cells were bound with R-Ag-NA647; only y-axis fluorescence shifts were observed. FSC thus served as a stable gate parameter, indicated by the blue bar, and was used for creating panel b. In f, both SSC and fluorescence increased due to R-Ag-NA647 (red). Etching caused a slight loss in signal from cells (upper-right population), attributed to the etching away of membrane-bound Ag, and a loss of events below the cell-minimum SSC at ∼106 counts was attributed to free R-Ag-NA647 or debris.