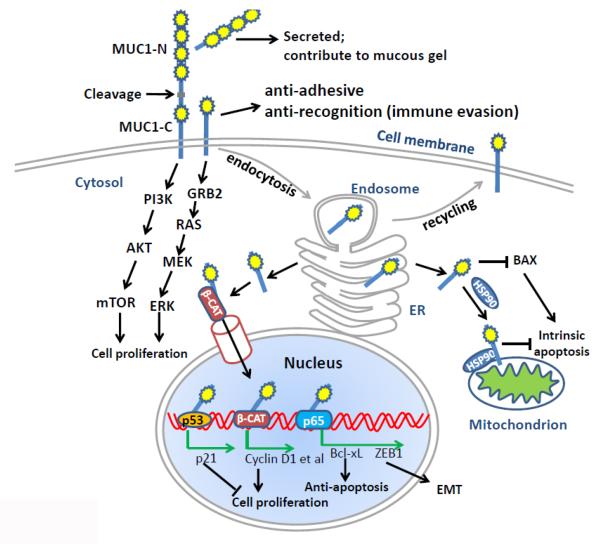
Figure 1. The complex role of MUC1 at different subcellular localizations in cancer.
MUC1 is cleaved at the SEA domain to generate two subunits, MUC1-N and MUC1-C. MUC1-N is secreted to extracellular compartments and contributes to the mucous gel. MUC1-C is a transmembrane subunit. The extracellular portion of MUC1-C is thought to play anti-adhesive and anti-recognition functions, which may be involved in the immune evasion. The intracellular portion of MUC1-C can interact with multiple signaling molecules such as PI3K p85 subunit and GRB2, activating AKT/mTOR and RAS/ERK pathways, respectively. The activation of these signaling pathways is important for cell survival and proliferation. MUC1-C constitutively undergoes endocytosis for receptor recycling. MUC1-C in the ER can be released to cytosol by the Sec61 translocon. The cytoplasmic MUC-C1 can be further imported into nucleus, where it associates with multiple transcriptional factors or nuclear receptors, such as p53, β-catenin and NF-κB p65, inducing the expression of targeted genes that are important to tumor cell proliferation or survival in the adverse microenvironment. Hsp90 can assist the transport of cytoplasmic MUC1-C onto mitochondria, where it suppresses intrinsic apoptosis. The cytoplasmic MUC1-C can also bind to proapoptotic protein BAX, antagonizing its function.