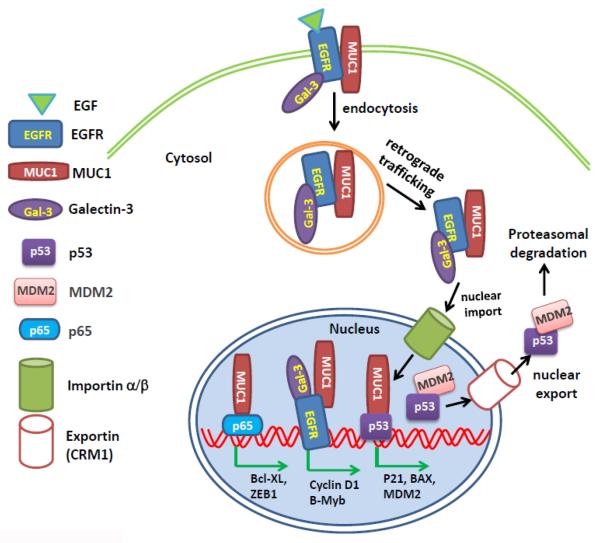
Figure 2. Schematic model of cross-regulation of protein subcellular localization of oncoproteins and tumor suppressor proteins.
EGFR is constitutively associated with MUC1 on the plasma membrane. On binding of epidermal growth factor, EGFR and MUC1 are endocytosed. Both proteins undergo retrograde trafficking through the endoplasmic reticulum and are imported into the nucleus via importin β1. Galectin-3 associates with EGFR to facilitate the nuclear import of MUC1 and EGFR [94]. The interaction of MUC1 and EGFR in the nucleus promotes nuclear EGFR accumulation and transcriptional activation, which induces the gene expression of cyclin D and B-Myb and promotes cell proliferation. Nuclear MUC1 also binds to NF-κB p65 and induces p65-mediated gene expression of Bcl-xL and ZEB1. The nuclear association between MUC1 and p53 induces transcription of the p53 promoter and expression of p21, Bax, and MDM2. MDM2 is a negative regulator of p53. It binds to p53 in the nucleus and assists the nuclear export of MDM2 to the cytosol, where p53 is sequestered and targeted for degradation. The coordination and antagonization between these oncoproteins and tumor suppressor proteins result in tumor cell survival, proliferation, and progression.