Figure 2.
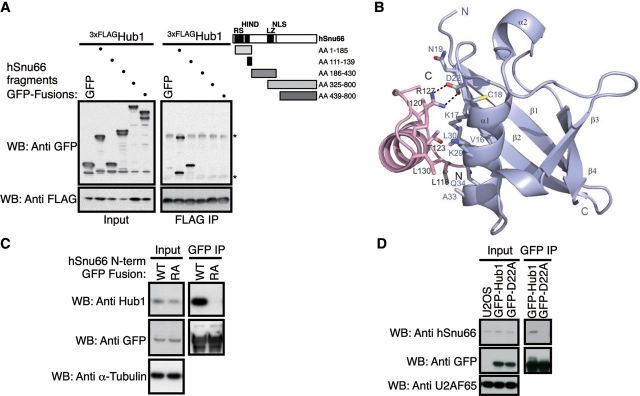
Molecular mode of interaction between human Hub1 and HIND. (A) Mapping of the Hub1 interaction domain in hSnu66 using FLAG-immunoprecipitation of 3xFLAG-Hub1 after co-expression of GFP-tagged hSnu66 truncations or free GFP in human cells. Immunoprecipitates were immunoblotted with anti-FLAG and anti-GFP antibodies (Asterisks indicate light and heavy chains). (B) Crystal structure of human Hub1 (blue) in complex with HIND peptide (pink) of hSnu66 shown as a ribbon plot with a resolution of 2.0 Å. The interaction between Hub1 and the α-helical HIND peptide is mediated through a salt bridge formed by D22 of Hub1 and R127 of HIND, strengthened by hydrophobic contacts involving aliphatic fragments of residues of hSnu66's HIND (L118, I120, T123, L126, R127 (Cβ and Cγ), L130, L132, L135) and the Hub1 interface (M1, V16, L17 (Cβ, Cγ, Cδ), C18, N19 (Cβ, Cγ), L29 (Cβ, Cγ, Cδ), L30, A33). (C) GFP-directed immunoprecipitation of GFP fused to the HIND containing N-terminal domain (aa 1–139) of wild-type hSnu66 (WT) or Hub1-binding-deficient HIND mutant (R127A). Immunoblot detection using antibodies directed against GFP, human Hub1, or α-tubulin (control). (D) Co-immunoprecipitation of Hub1 with hSnu66 depends on the HIND interaction interface. GFP immunoprecipitation from U2OS cells stably expressing GFP-tagged Hub1 WT or hSnu66 binding mutant Hub1 D22A. Immunoblots were probed with anti-GFP and anti-hSnu66 antibodies with anti-U2AF65 serving as a loading control.
