Fig. 1.
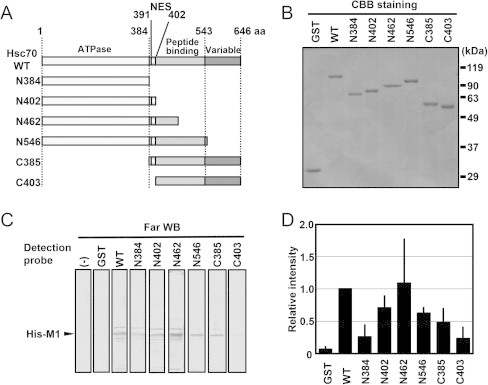
Association of Hsc70 with M1 via the NES region in the N-terminal half of the peptide-binding domain. (A) Schematic representations of Hsc70 deletion mutants. (B) Purified GST-fused Hsc70 deletion mutants. One microgram-equivalent of the purified GST-fused Hsc70 mutants was loaded onto 10% SDS–PAGE, followed by CBB staining. (C) Far-western blotting assay. The far-western blotting assay was performed in accordance with a previously reported method [24]. In brief, the crude cell lysate of E. coli that expressed His–M1 was separated by 10% SDS–PAGE, transferred onto a PVDF membrane, and denatured with 6 M guanidine HCl in TBST for 10 min. After stepwise renaturating, the membrane was incubated without [“(−)”] or with 1 μg/mL of each purified GST or the series of GST–Hsc70 deletion mutants (see panels A and B). Specific signals were detected by western blotting using anti-GST antibody. Representative data from three independent experiments are shown. (D) The band intensity was measured using ImageJ software. Data represent the means and standard deviations from three independent experiments.
