Abstract
Background
Human immunodeficiency virus type 1 (HIV-1)-seropositive patients are at a high risk for the development of a variety of acute and chronic renal diseases. Most patients with HIVAN are of African descent, presenting late in the course of their HIV-1 infection. The only reliable test to establish or rule out the presence of HIVAN (HIV associated nephropathy) is renal biopsy. The most common lesion associated with HIV is a focal segmental glomeruloscelerosis, but several times, other biopsy findings may also be seen. Our patient had lupus nephritis like pathology picture. The therapeutic agents with the most promise are angiotensin-converting enzyme inhibitors and antiretroviral medications. Role of steroids are less well-defined although they have been used with success many times.
Case Details
Our patient was a young male who presented with a pulmonary renal syndrome like picture and wasting. On evaluation, he was found to be HIV-1 positive, and renal biopsy showed lupus nephritis like pathological picture. The patient was treated with HAART (Highly active anti retroviral therapy) , steroids and ACE inhibitors and showed an excellent response.
Conclusion
The case highlights the fact that immune mediated glomerulonephritis, although rare, can be the presenting feature of HIV infection and can be controlled, if not cured, with proper treatment.
Keywords: HIV, Glomerulonephritis, HIV associated nephropathy
Introduction
The association between HIV (Human Immunodeficiency Virus) and renal disease was first reported in 1984 by investigators in New York City and Miami. They reported a series of HIV-1-seropositive patients who developed a renal syndrome characterized by progressive renal failure and proteinuria (1). The most common kidney biopsy finding was focal segmental glomerulosclerosis (FSGS) (2). During the next several years, the existence of a specific HIV associated nephropathy (HIVAN) was debated, in part, because of its similarity with heroin nephropathy and the frequent occurrence of intravenous drug use in this population (4). The study by Wearne and colleagues in 2011, which included the largest group of patients of African ancestry with HIVAN studied so far, describes renal histological variants of HIVAN, and suggests that antiretroviral therapies improve the clinical outcome of all HIV-associated renal diseases. They could identify only 9 cases of immune complex glomerulopathy of the 221 biopsies studied.We present a case of a young Asian male with HIV associated nephropathy with rare lesion of immune mediated proliferative glomerulonephritis who responded to steroids, HAART (Highly Active Antiretroviral Therapy) and ACE inhibitors (angiotensin-converting-enzyme inhibitors).
Case Details
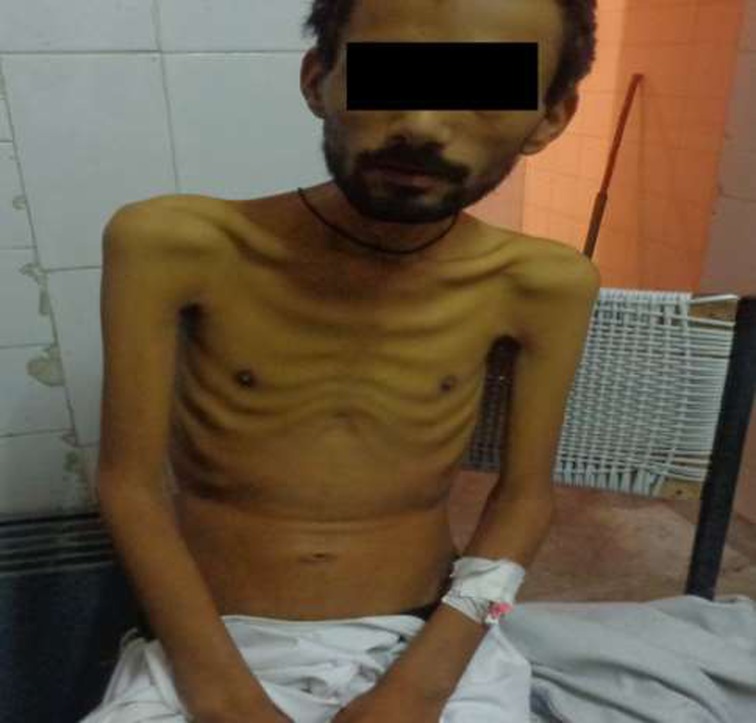
A 25 years old male from the Kargil area of North India presented with cough, expectoration and pedal edema of one month duration. There was associated high grade fever and minimal hemoptysis. Also, there was a history of chronic diarrhea in the form of passage of watery loose stools 3 to 4 times daily for one year. The patient had a significant weight loss. On examination patient was found to be emaciated with a body mass index of 16.There was mild pedal edema. There was no hypertention. Chest examination revealed crepiations and bronchial breathing in the left mammary area.
Chest X-ray showed a left upper and mid zone consolidation. HRCT chest showed patchy cavitary consolidation of the upper lobe of left lung. Urine examination showed an active sediment and 24 hours urinary examination revealed nephrotic range proteinuria (4gm/24 hrs). The patient dropped his urine output and the serum creatinine was worsening from the initial of 1.1 to 3.2. A renal biopsy was planned which showed immune mediated proliferative glomerulonephritis (mesangial and endocapillary proliferation) with crescents in 33 percent of the glomeruli examined exhibiting segmentally thickened capillary walls (with double contours) and nodular mesangial expansion with presence of subendothelial and mesangial non congophilic , non argyrophyllic , fuchsinophilic deposits. Direct Immunofluoresence of the same revealed capillary wall and mesangial deposits of immunoglobulins (Ig G, Ig M) and complement product C3. With this histopathologic and clinical picture the patient was evaluated for a possible pulmonary renal syndrome. Simultaneously, the patient was put on treatment for community acquired pneumonia with ceftriaxone and azithromycin. Work up for all the pulmonary renal syndromes proved to be negative with negative ANCA, anti GBM. Also, as the renal biopsy features favored lupus nephritis like picture, ANA, anti dsDNA, anti smith antibodies, were sought but all were negative. Further, the patient's clinical profile was also against the diagnosis of systemic lupus nephritis. Tubercular work up (mantoux test, sputum for acid fast bacilli, sputum culture, urine for acid fast bacilli) was negative. Stool examination also did not help the diagnosis as it showed only non-specific inflammatory cells and no organism of significance could be grown. Hemogram showed pancytopenia. It was at this time the patient's kidney function further deteriorated and was put on pulse steroids emperically (1g of methylprednisolone intravenously given daily, for three days) which did help in recovering the kidney function. In view of chronic diarrhea, emaciation , and the high risk sexual behavior (heterosexual) an ELISA was done for anti-HIV (Human immunodeficiency virus) antibodies which was positive for HIV-1 and was confirmed with western blot. CD4 cell count was found to be 175 and HIV viral load was 100,000 copies per ml. Virological screening for hepatitis B and C viruses was negative.
With this history, examination and investigations a final diagnosis of HIV AIDS (Acquired Immunodeficiency Syndrome), HIV associated nephropathy, HIV associated pancytopenia and community acquired pneumonia was made. Treatment was initiated with highly active antiretroviral therapy [HAART; elvitegravir (150 mg) + cobicistat (150 mg) + emtricitabine (200 mg) + tenofovir (300 mg) qd].Oral steroids were also prescribed(prednisone 60 mg qd for 1 month and subsequently tapered) as the patient had initially shown a good response to steroids. ACE inhibitors were introduced once the renal parameters had stabilized after around 4 to 5 weeks. Antibiotics were given for a total of 14 days after which there was clinical improvement in chest signs and symptoms. Subsequently, cryptosporidium was isolated in the stools of the patient, and the patient was put on a lactose free diet. Nutritional supplement with zinc and glutamine was given. Nitazoxanide PO 500 mg every 12 hour for 3 days was given along with HAART.As in the case with most HIV cryptosporidiosis, the diarrhea responded only after HAART had started to act, after around one month.
At 12 months follow-up the patient improved significantly. HIV viral load is less than 500 per ml, the proteinuria is minimal, the chronic diarrhea has been treated, the patient has significant weight gain and CD4 count is 650.
Discussion
In the past 18 years since HIVAN was first described, much has been published regarding the epidemiology, pathogenesis, and treatment of this disease. Despite these advances, however, HIVAN continues to be a serious cause of renal failure in the United States and other countries with large populations of African descent (2). HIV associated nephropathy (HIVAN) is usually diagnosed in patients who have been HIV-1-seropositive for several years and most patients have low CD4 counts and/or other criteria for the diagnosis of AIDS (4). In the post- highly active antiretroviral therapy (HAART) era, however, more patients are being identified who present earlier in the course of HIV infection with CD4 counts above 200 (5). HIVAN is often characterized clinically by the presence of proteinuria, but not necessarily in the nephrotic range (6, 7). Most patients have moderate to severe renal insufficiency at the time of diagnosis (2), although a cohort of patients with HIVAN and mild renal insufficiency has been recently described (6). Several types of renal disease seem to be directly or indirectly caused by HIV: classic HIVAN, HIV-associated thrombotic microangiopathy, and HIV-associated, immune-mediated glomerulonephritides.
Classic HIVAN is a syndrome caused by focal sclerosing glomerulopathy with severe proteinuria, renal failure, and rapid progression to ESRD. It has become the most common cause of ESRD in HIV-1-seropositive patients. HIVAN primarily occurs in patients of African descent (8), suggesting a genetic predisposition to the disease. Duffy antigen/receptor for chemokines has been controversially discussed as a candidate gene involved in the development of HIVAN (9).
The estimated prevalence of HIVAN has ranged from 3.5% in clinical studies to 12% in autopsy studies (10). Because HIVAN typically occurs late in the course of HIV-1 infection, risk factors for the development of HIVAN include a CD4 cell count <200 cells/mm3 and a high viral burden. Clinical features of this syndrome include advanced renal failure and proteinuria (the protein level is often-but not necessarily-at a nephrotic level, a lack of peripheral edema despite the severe loss of protein, and, frequently, enlarged kidneys visible on renal ultrasound. Renal biopsy is the only means to establish the diagnosis of HIVAN. Characteristic histological findings include collapsing focal and segmental glomerulosclerosis, tubular epithelial atrophy with microcystic dilatation of the tubules, and lymphocytic interstitial infiltration. Viral infection of renal cells seems to play an important role in the pathogenesis of HIVAN (10).
Thrombotic microangiopathy: Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura present a spectrum of diseases characterized by haemolytic anemia, thrombocytopenia, and renal insufficiency and clinical features such as fever and neurological manifestations. Several reports have linked thrombotic microangiopathy HIV infection, suggesting that HIV proteins may mediate endothelial dysfunction, leading to platelet deposition in the microvasculature (11).
Immune complex-mediated glomerulonephritis: A multitude of immune complex-mediated glomerulonephritides have been reported as causes of chronic kidney disease in HIV infected patients. The prevalence of HIV-associated, immune complex-mediated glomerulonephritides has been estimated to be 15%–80% (10). A study of 60 biopsy specimens found that some form of immune complex-mediated glomerulonephritis was present in 37% of biopsy specimens, sometimes concordant with HIVAN-like changes. The findings have been into 4 categories: immune complex-mediated glomerulonephritis, IgA nephritis, mixed sclerotic/inflammatory disease and lupus-like syndrome (12). Immune complex-mediated glomerulonephritis may present as postinfectious glomerulonephritis, membranous nephritis, IgA nephritis, fibrillary glomerulonephritis, immunotactoid glomerulopathy, and membanoproliferative glomerulonephritis (13). IgA nephritis seems to be more prevalent among European patients, with mesangial IgA deposits being detected in 7.75% of all HIV-infected patients in a postmortem study (14). A recent report estimated that the prevalence of lupus-like nephritis, characterized by immunoglobulin (IgG, IgA, and IgM) and complement (C3 and C1q) deposits, in the absence of serologic markers for systemic lupus erythematodes, was 17% (10). IgA nephritis seems to be more prevalent among European patients, with mesangial IgA deposits being detected in 7.75% of all HIV-infected patients in a postmortem study (14). A recent report estimated that the prevalence of lupus-like nephritis, characterized by immunoglobulin (IgG, IgA, and IgM) and complement (C3 and C1q) deposits, in the absence of serologic markers for systemic lupus erythematodes, was 17% (15). Our patient, an Asian, was a case HIVAN of immune mediated glomerulonephritis with immune deposits of Ig G, Ig M and C3 complement product.
Patients with HIV-associated, immune complex-mediated glomerulonephrits seem to benefit from treatment with angiotensin-converting enzyme inhibitors, glucocorticoids and antiretrovirals (16, 17). Our patient was also managed with steroids, ACE inhibitors, HAART to which he showed promising response.
Wearne et al. in a study of 221 HIV-positive renal biopsies found a spectrum of renal histologies of which HIV-associated nephropathy (HIVAN) was the most common histology. Combined antiretroviral therapy reduced the mortality in those with any feature of HIVAN by 57% (18). They identified HIVAN plus immune complex GN (HIVAN plus ICGN) in nine biopsies which had immune complex deposition with a ‘ball-in-cup’ appearance. In all of these cases, there was at least one other defining feature of HIVAN. The ball-in-cup deposit was most commonly associated with C3 positivity.
The epidemiology of HIVAN reveals a strikingly skewed ethnic distribution. Among HIV-infected individuals, almost all cases of HIVAN occur in patients of African descent (19). This skewed ethnic prevalence has been confirmed in European studies and is not explained by the mode of viral transmission (20, 21). Moreover, not all African populations seem to have the same predisposition to HIVAN. A screening of 126 Ethiopians who had been HIV infection and emigrated to Israel revealed that none of the individuals met clinical criteria for HIVAN, as defined by proteinuria >2 g/d [22]. Interindividual variation, racial differences, and familial clustering of kidney disease strongly suggest the existence of HIVAN-specific genetic susceptibility factors that are unmasked in the setting of HIV infection. Although there was an initial suggestion that a polymorphism in the promoter of the Duffy antigen/receptor for chemokines may be involved in susceptibility to HIVAN (23), this has not been confirmed in other studies (24). In addition to expression of viral genes, a permissive genetic background is required for manifestation of the HIVAN phenotype (25). A genome-wide analysis of linkage has identified a locus on mouse chromosome 3A1–3 that influences the HIVAN phenotype (named HIVAN1 locus) (25). It is interesting that this locus is syntenic to human chromosome 3q25–27, an interval that shows suggestive evidence of linkage to human diabetic and hypertensive nephropathies (26).
HIV associated Immune-complex nephropathy is associated with advanced HIV disease. ESRD incidence is lower in HIV associated Immune Complex nephropathy patients compared with those with HIVAN. Unlike HIVAN, combined antiretroviral therapy use is not associated with the incidence of ESRD in Immune complex nephropathy (27).
Findings from light microscopy of kidney biopsy tissue are diagnostic in most cases of HIVAN. The most common histologic light microscopy finding is a collapsing form of focal segmental glomerulosclerosis. The glomerular capillary tuft is collapsed and may be segmentally or globally sclerosed. Visceral epithelial cells are hypertrophied and form a characteristic pseudocrescent in the Bowman space. Tubulointerstitial scarring, atrophy, and marked dilatation of the tubules are usually present (28). In contrast, Immune complex glomerulonephritis has various histological presentations like Mesangial proliferative glomerulonephritis, Membranous nephropathy, IgA nephropathy, Lupus-like nephritis, Membranoproliferative glomerulonephritis, Post-infectious glomerulonephritis. The majority are of Caucasian origin. Deposition of immune complexes containing HIV antigens has been occasionally demonstrated in renal tissue from patients with immune mediated glomerulonephritis suggesting a direct viral effect (29). In addition, specific implication of co-infection with hepatitis B and C virus has also been suggested, mostly for mesangioproliferative and membranoproliferative glomerulonephritis (30).
It is important to rule out hepatitis B(HBV) and C(HCV) co-infection in patients with immune-complex glomerulopathy. In some patients co-infected with HIV and HCV, the development of immune complex glomerulonephropathy may dominate the clinical course of the disease. The occurrence of immune complex glomerulonephropathy among black patients at risk for HIVAN may be related to the relatively high prevalence of HCV infection among intra venous drug users in this group (31). The pathogenesis of HBV-related kidney disease is hypothesized to involve the deposition of HBeAg in glomerular capillaries (32). Suppression of HBV replication with interferon or lamivudine has been associated with remission of kidney disease in some, but not all, cases of HBV-related immune complex kidney disease (33).
The case highlights the fact that immune mediated glomerulonephritis, although rarer, can be the presenting feature of HIV infection. With proper treatment it can be controlled if not cured.
Figure 1.
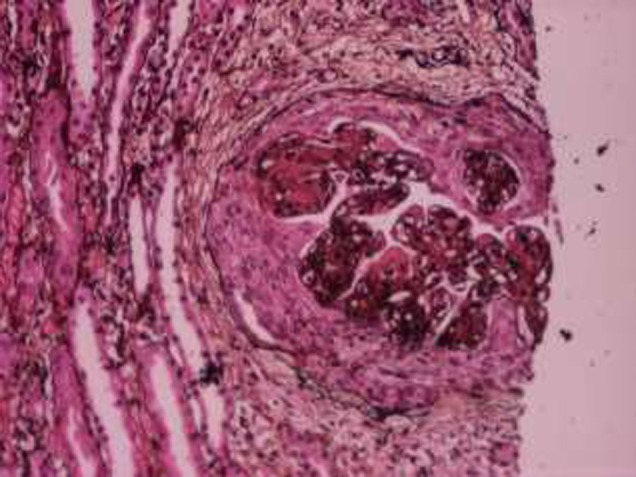
A histopathological picture showing mesangial and endocapillary glomerulonephritis
Figure 2.

HRCT chest showing a left upper lobe consolidation
Figure 3.
Image of the patient showing the emaciated appearance before the treatment
Figure 4.
Image of the patient 12 months after treatment
References
- 1.Gardenswartz MH, Lerner CW, Seligson GR, Zabetakis PM, Rotterdam H, Tapper ML, Michelis MF, Bruno MS. Renal disease in patients with AIDS: a clinicopathologic study. Clin Nephrol. 1984;21:197–204. [PubMed] [Google Scholar]
- 2.Ross MichaeL J, Klotman: Paul E. The spectrum of renal histologies seen in HIV with outcomes prognostic indicators and clinical correlations. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/GFR702. Recent Progress in Wearne N, Swanepoel C, Boulle A, et al. [DOI] [PubMed] [Google Scholar]
- 3.HIV-Associated Nephropathy. J Am Soc Nephrol. 2002;13:2997–3004. doi: 10.1097/01.asn.0000040750.40907.99. [DOI] [PubMed] [Google Scholar]
- 4.Mazbar SA, Schoenfeld PY, Humphreys MH. Renal involvement in patients infected with HIV: Experience at San Francisco General Hospital. Kidney Int. 1990;37:1325–1332. doi: 10.1038/ki.1990.118. [DOI] [PubMed] [Google Scholar]
- 5.Winston JA, Klotman ME, Klotman PE. HIVassociated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int. 1999;55:1036–1040. doi: 10.1046/j.1523-1755.1999.0550031036.x. [DOI] [PubMed] [Google Scholar]
- 6.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D'Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primaryinfection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 7.Burns GC, Paul SK, Toth IR, Sivak SL. Effect of angiotensinconverting enzyme inhibition in HIV-associated nephropathy. J Am SocNephrol. 1997;8:1140–1146. doi: 10.1681/ASN.V871140. [DOI] [PubMed] [Google Scholar]
- 8.Carbone L, D'Agati V, Cheng JT, Appel GB. Course and prognosis of human immunodeficiency virus-associated nephropathy. Am J Med. 1989;87:389–395. doi: 10.1016/s0002-9343(89)80819-8. [DOI] [PubMed] [Google Scholar]
- 9.Bourgoignie JJ, Ortiz-Interian C, Green DF, Roth D. Race, a cofactor in HIV-1-associated nephropathy. Transplant Proc. 1989;21:3899–3901. [PubMed] [Google Scholar]
- 10.Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of Duffy antigen receptor expression in children with renal disease. Kidney Int. 1999;55:1491–1500. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 11.Roling J, Schmid H, Fischereder M, Draenert R, Goebel:HIV-Associated F D. Renal Diseases and Highly Active Antiretroviral Therapy-Induced Nephropathy. Clinical Infectious Diseases. 2006;42:1488–1495. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- 12.Alpers CE. Light at the end of the TUNEL: HIV-associated thrombotic microangiopathy. Kidney Int. 2003;63:385–396. doi: 10.1046/j.1523-1755.2003.00743.x. [DOI] [PubMed] [Google Scholar]
- 13.Nochy D, Glotz D, Dosquet P, et al. Renal disease associated with HIV infection: a multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant. 1993;8:11–19. doi: 10.1093/oxfordjournals.ndt.a092263. [DOI] [PubMed] [Google Scholar]
- 14.Kimmel PL, Barisoni L, Kopp JB. Pathogenesis and treatment of HIVassociated renal diseases: lessons from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Ann Int Med. 2003;139:214–227. [PubMed] [Google Scholar]
- 15.Beaufils H, Jouanneau C, Katlama C, Sazdovitch V, Hauw JJ. HIVassociated IgA nephropathy: a post-mortem study. Nephrol Dial Transplant. 1995;10:35–38. [PubMed] [Google Scholar]
- 16.Haas M, Kaul S, Eustace JA. HIV-associated immune complex glomerulonephritiswith “lupus-like” features: a clinocopathological study of 14 cases. Kidney Int. 2005;67:1381–1390. doi: 10.1111/j.1523-1755.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 17.Tabechian D, Pattanaik D, Suresh U, Cohn SE, Nadasdy T. Lupuslike nephritis in an HIV-positive patient: report of a case and review of the literature. Clin Nephrol. 2003;60:187–194. doi: 10.5414/cnp60187. [DOI] [PubMed] [Google Scholar]
- 18.Gorriz JL, Rovira E, Sancho A, Ferrer R, Paricio A, Pallardo LM. IgA nephropathy associated with human immunodeficiency virus infection: antiproteinuric effect of captopril. Nephrol Dial Transplant. 1997;12:2796–2797. doi: 10.1093/ndt/12.12.2796. [DOI] [PubMed] [Google Scholar]
- 19.Cantor ES, Kimmel PL, Bosch JP. Effect of race on expression of acquired immunodeficiency syndrome-associated nephropathy. Arch Intern Med. 1991;151:125. [PubMed] [Google Scholar]
- 20.Nochy D, Glotz D, Dosquet P, Pruna A, Lemoine R, Guettier C, Weiss L, Hinglais N, Idatte JM, Mery JP. Renal lesions associated with human immunodeficiency virus infection: North American vs. European experience. Adv Nephrol Necker Hosp. 1993;22:269–286. [PubMed] [Google Scholar]
- 21.Husain M, D'Agati VD, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: A characteristic feature of HIVAN. AIDS. 2005;19:1975–1980. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- 22.Behar DM, Shlush LI, Maor C, Lorber M, Skorecki K. Absence of HIV-associated nephropathy in Ethiopians. Am J Kidney Dis. 2006;47:88–94. doi: 10.1053/j.ajkd.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of Duffy antigen receptor expression in children with renal disease. Kidney Int. 1999;55:1491–1500. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 24.Woolley IJ, Kalayjian R, Valdez H, Hamza N, Jacobs G, Lederman MM, Zimmerman PA. HIV nephropathy and the Duffy antigen/receptor for chemokines in African Americans. J Nephrol. 2001;14:384–387. [PubMed] [Google Scholar]
- 25.Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D'Agati VD, Klotman PE, Lifton RP. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci U S A. 2004;101:2488–2493. doi: 10.1073/pnas.0308649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterholm AM, He B, Pitkaniemi J, Albinsson L, Berg T, Sarti C, Tuomilehto J, Tryggvason K. Genome-wide scan for type 1 diabetic nephropathy in the Finnish population reveals suggestive linkage to a single locus on chromosome 3q. Kidney Int. 2007;71:140–145. doi: 10.1038/sj.ki.5001933. [DOI] [PubMed] [Google Scholar]
- 27.Foy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, Atta MG. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy . Clin J Am Soc Nephrol. 2013;8(9):1524–1532. doi: 10.2215/CJN.10991012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KT, Papeta N, Martino J, et al. Accelerated development of collapsing glomerulopathy in mice congenic for the HIVAN1 locus. Kidney Int. 2009;75(4):366–372. doi: 10.1038/ki.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimmel PL, Phillips TM, Ferreira-Centeno A, et al. Brief report: idiotypic IgA nephropathy in patients with human immunodeficiency virus infection. N Engl J Med. 1992;327:702–706. doi: 10.1056/NEJM199209033271006. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SD, Kimmel PL. Immune complex renal disease and human immunodeficiency virus infection. Semin Nephrol. 2008;28:535–544. doi: 10.1016/j.semnephrol.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Stokes MB, et al. Immune complex glomerulonephritis in patients coinfected with human immunodeficiency virus and hepatitis C virus. Am J Kidney Dis. 1997;29(4):514–525. doi: 10.1016/s0272-6386(97)90332-2. [DOI] [PubMed] [Google Scholar]
- 32.Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324(21):1457–1463. doi: 10.1056/NEJM199105233242103. [DOI] [PubMed] [Google Scholar]
- 33.Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68(4):1750–1758. doi: 10.1111/j.1523-1755.2005.00591.x. [DOI] [PubMed] [Google Scholar]