Abstract
Objectives
This study was an in-vitro evaluation of different brands of paracetamol and cotrimoxazole tablets, used or found in Malawi, based on Pharmacopoeia standards, in order to ascertain the existence and extent of substandard medicines in Malawi and to give an overview of their distribution in the public and private sectors.
Methodology
A cross-sectional analytical study was conducted using 11 samples each of paracetamol and cotrimoxazole tablets. Stratified random sampling was used to collect samples. Samples were analyzed using HPLC and Spectrophometric methods as outlined in the BP-2007 and USP-32 at the National Drug Quality Control Laboratory (NDQCL)-Lilongwe (under Pharmacy Medicines and Poisons Board-PMPB) and Orient Pharma Co. Ltd of Taiwan. The results were analyzed using Epi Info.
Results and discussion
Fifty percent of samples (n=22) were not registered in the country by the PMPB as required by the PMP Act with the majority of those coming from public health facilities. All paracetamol and cotrimoxazole samples complied with identification tests using spectrophotometric and HPLC method. Overall, 27.3% of samples failed to meet the BP-2007 standards for Active Ingredient content, while 22.7% of the samples failed the Friability test. The results from Malawi are similar in magnitude to those within surrounding countries in Africa.
Conclusion
This pilot study provides objective evidence to show that substandard and unregistered paracetamol and cotrimoxazole are present and being used in Malawi, and thus posing a considerable hazard to public health in Malawi. PMPB, together with the Ministry of Health, must continue to develop a quality assurance system to ensure that medicines are randomly and routinely checked.
Introduction
The Malawi Government through the Ministry of Health and Pharmacy Medicines and Poisons Board (PMPB) adopted the Essential Drugs Concept in its National Drug Policy.1 The overall aim of the policy is: to contribute to the attainment of quality health of the Malawian population through equitable access to good quality, safe and efficacious drugs at affordable cost and rational use1. Malawi as a country has been focusing much of its attention in the area of rational drug use, while not much information is available concerning the quality of the essential drugs on the Malawian market.
A fundamental quality attribute for all pharmaceutical preparations is the requirement for constant dose of drug between individual tablets. In practice, small variations between individual preparations are accepted and the limits for this variation are defined as standards in official pharmacopoeias like the British Pharmacopoeia (BP) or the United States of America Pharmacopoeia (USP).2 There are several ways in which the quality of medicines can be assessed (which can either be In-vitro or In-vivo). In-vitro methods are procedures employing test apparatus and equipment in testing medicines without involving laboratory animals or humans.2 In-vivo methods are more complex studies involving human subjects or laboratory animals mainly in assessing bioequivalence of different brands.2 The in-vitro method is able to assess the impact of the physical and chemical properties of the drug, drug stability, and large-scale production of the drug and drug product on the biologic performance of the drug.
If a drug, upon laboratory testing in accordance with the specifications it claims to comply with, fails to meet the specifications, then it is classified as a substandard drug. Substandard drugs are drug products that do not meet quality specifications set for them and they may either be genuine or counterfeit medicinal products.3 The World Health Organization (WHO) defines a counterfeit medicine as “a medicine which is deliberately and fraudulently mislabeled with respect to identity and/or source3. Counterfeiting can apply to both branded and generic products and counterfeit products may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging.”4 There are several reports on the presence of medicines or drugs of doubtful quality (substandard medicines) on the market in both developed and developing countries.5–7
Consequences of substandard medicines include: reduction in bioavailability as a result of reduction in dissolution of active ingredient; the increasing drug-resistance among some of the world's most deadly infectious diseases, including malaria, tuberculosis and HIV/AIDS. The reason is that most counterfeit, substandard or degraded medicines contain incorrect levels of a drug's active ingredient, which causes the weaker strains of the causal agent to be killed off while allowing the drug-resistant strains to multiply and adapt.8
The other consequence of substandard drugs is the increase in cost of treatment of patients due to resistance as a result of using substandard medicines. This increase in cost will directly affect government as it is the major funding agency for public health facilities in Malawi. The same increase in cost will be felt by the patients and their guardians in terms of long stay in hospitals and in addition to direct payment in hospital fees.
Data about substandard medicines, from other countries,5–7 is available but there is lack of available data on the Malawian market regarding the quality of those medicines. The National Drug Quality Control Laboratory (NDQCL) (under PMPB) does routine testing of drugs when they are to be registered in the country, but does not currently do post marketing testing and surveillance. A sample report of the PMPB July 2006 to June 2007 showed that out of 472 samples tested for the Central Medical Stores-Malawi, 14 samples failed the test. Out of 213 products for which registration in Malawi was sought, 4 products failed the test.9 Overall 3% of the products failed the test, and this represents only those drugs for government health facilities, while the majority of drugs entering the country are not tested. As such much information of substandard medicines in Malawi is not available. As such there is a lack of adequate data to ascertain the existence and extent of substandard drugs in Malawi. Thus their use may result in reduction in bioavailability and an increase in drug-resistance to such drugs on the market.
The study was aimed at providing detailed information on the quality (that is: active drug content, in-vitro availability-disintegration and dissolution, and friability) of paracetamol and co-trimoxazole available as tablet formulations on the Malawian market. Paracetamol and co-trimoxazole are essential drugs and the Ministry of Health (Malawi) has listed them as vital drugs in the Malawi Standard Essential Medicines List, which must be available at all times from Health Centre level to Central Hospital level. Thus, the study aimed at establishing the existence of substandard medicines on the Malawian market; serve as a guide for future studies to quantify the extent of the problem in Malawi; and help policy makers regulate the availability of the substandard medicines or drugs.
Materials and Methodology
This was a cross-sectional analytical study conducted in Blantyre, at the College of Medicine with laboratory analysis of the drugs conducted in Lilongwe at PMPB's National Drug Quality Control Laboratory and afterwards sent to Orient Pharma in Taiwan for Dissolution Test and HPLC analysis.
The drugs were sampled from various Government Health Facilities, Private or Retail pharmacies, Illegal Drug vendors, and ordinary groceries or shops using a multi-stage stratified random sampling. The country was divided into three regions and in each region one district was selected. In each selected district samples were collected from randomly selected public and private sector health providers. Table 1 shows the detailed information on the samples that were assayed. A total of 11 different brands of paracetamol and 11 different brands of Cotrimoxazole tablets were assayed, making a combined total of 22.
Table1.
Demographic information of collected samples.
| Public Health Facility | Private Pharmacies | Illegal Vendors | Total | |
| Paracetamol samples |
6 | 4 | 1 | 11 |
| Cotrimoxazole samples |
5 | 4 | 2 | 11 |
| Total number of samples |
11 | 8 | 3 | 22 |
The drugs were anonymously purchased (or randomly selected in public health facilities) in their original package as supplied by the manufacturers, and only one package of a particular brand was bought from any selected retail Pharmacy. All formulations sampled had a remaining stated shelf life of at least 1 year at the time of sampling. All the samples were coded and manufacturer's identity has been excluded for ethical reasons.
Checking of registration status was done by checking the label of the bottle and also by looking at the registration status from the PMPB's Registered List of Products. Laboratory technicians from PMPB's National Drug Quality control Laboratory helped the Investigator in the initial pharmaceutical analysis in Malawi and Laboratory technicians/technologists of Orient Pharma company under the guidance of Professor David H.W. Cheng of Taipei Medical University did the final part of the analysis (which included dissolution, HPLC analysis and validation of the procedures) in Taiwan.
Pure Paracetamol and Sulphamethoxazole and Trimethoprim standards were provided by the PMPB-Malawi and Orient Pharma in Taiwan. All reagents used during drug assay were of HPLC grade.
All drugs were assayed for the drug content immediately after purchase. The tested tablets were randomly selected from packages of the same batch. The assays were repeated three times and the results presented are the mean of three determinations. All drugs were assayed according to methods outlined in the individual drug monographs of the British Pharmacopoeia 2007, except for Dissolution method which used United States Pharmacopoeia-32.
Data and Statistical analysis
Microsoft Windows Excel 2003 and Epi Info were used in the data entry and analysis respectively. Analysis of variance (ANOVA) was used to compare the results between Public Health Facilities, Private Pharmacies and Illegal Vendors with 95% confidence interval (thus p=0.05).
Results
On registration of samples, it was found out that 50% (n=22) of the samples were not registered as of 30th June 2009 (six paracetamol samples and five co-trimoxazole samples) by the PMPB as required by the law, with public health facilities having 72.7% (n=11) of samples not registered as compared to 28.57% (n=8) for private pharmacies and 33.3% (n=3) for Illegal vendors.
Also, all paracetamol and Cotrimoxazole (Sulfamethoxazole and Trimethoprim) tablets complied with identification of active ingredient as outlined in the BP 2007 and USP 32.
On individual tablet weight uniformity, only one product failed to meet the required standard, representing 5% (n=22). ANOVA analysis of Uniformity of Tablet Weight of samples did not show a significant difference (p=0.001) between public health facilities, private pharmacies and Illegal vendors.
The content of paracetamol active ingredient in the samples ranged from 94.57 to 98%, thus some falling short of the acceptance limit for paracetamol of 95–105% in the BP. Ten paracetamol tablet brands met the required standard with only one paracetamol tablet formulation failing to meet the standard of content of active ingredient, thus representing 9.1% of paracetamol samples.
The content of sulfamethoxazole in the samples ranged from 91.04–107.3%, while that of Trimethoprim was 65.57–110.13%. The acceptance range for both sulfamethoxazole and Trimethoprim in BP 2007 is 92.5–107.5%. Five co-trimoxazole brands failed to meet the required standards thus representing 45.5% of co-trimoxazole samples, with one of the brand having too much Trimethoprim content (110.13%).
Combination of paracetamol and cotrimoxazole samples showed that six tablet formulations failed to meet the BP 2007 standards of Active Ingredient content, thus representing 27.3%.
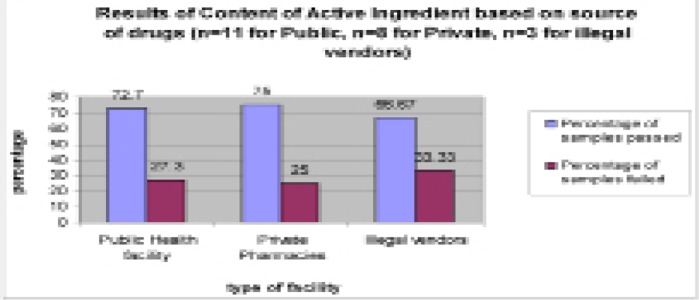
The results of active ingredient based on sources of drugs showed that 27.3% (n=11) samples from public health facilities did not meet the required standard, while for private pharmacies and illegal vendors it was 25% (n=8) and 33.3% (n=3), see figure 1 below. The differences in failure rate based on source of drugs was not statistically significant (p=0.001).
Figure 1.
Results of content of active ingredient based on Type of Facility
All paracetamol and cotrimoxazole brands complied with the Disintegration test.
Only one formulation failed to meet the BP 2007 or USP-32 requirements for Dissolution test, which is a release of more than 80% of paracetamol within 30 min and more than 70% for cotrimoxazole.
A product passes Friability Test if the friability percent loss is less than 1%. There were three paracetamol preparations and two co-trimoxazole preparations which failed the test. On friability, 22.7% (n=22) of all tablet formulations failed the test, all of them were from public health facilities. ANOVA analysis of Friability of samples showed a significant difference (p=0.001) in the Friability failure rate. Public health facilities had a failure rate of 45.5% (n=11) of samples while private pharmacies and Illegal vendors did not have any friability failure.
Discussion
From the study, it had been observed that half of the sampled products were not registered with PMPB in Malawi with the MOH appearing to be the biggest source of the problem. This may be due to laxity by the Central Medical Stores staff when tendering or purchasing medicines for the public sector. Donors influence could also have been a contributing factor to this, since majority of donors put strict restrictions on where to buy the medicines when using their funds, as such CMS and MOH may be forced to procure unregistered medicines. While inadequate human resource at the PMPB may have contributed to some of unregistered drugs which were found by illegal vendors and private Pharmacies. Registration of Medicines in a country is a major pre-requisite before the medicine can be used by the general population. Registration ensures that the medicines have been thoroughly checked in terms to safety, quality and efficacy. A study conducted by Prazuck. T et al. in Northern Myanmar found out that 14.3% of the samples (n=21) displayed the official registration number/label10 while in this study, none of the products had its registration number displayed. If the maintenance of a healthy nation is to be achieved then Registration of medicines by PMPB is an important process which should not be ignored. By not displaying the registration number on the label, it becomes difficult for an ordinary consumer of the medicines or the health professionals to know if the product they are buying is an original or substandard or even counterfeit.
A fundamental quality attribute for all pharmaceutical preparations is the requirement for constant dose of drug between individual tablets. In practice, small variations between individual preparations are accepted and the limits for this variation are defined as standards in official pharmacopoeias like the BP and USP11,12. This study has demonstrated high overall failure rates for content uniformity of the Co-trimoxazole and Paracetamol tablets at 27.3% (n=22). Several studies have reported on low content of active ingredient. Gaudiano, M. et al. (2007) reported one sample as having low quantity of active ingredient in a study carried out in Congo, Burundi and Angola13. Ogwal-Okeng et al. found that 39% of chloroquine in Uganda failed the active ingredient content test with 11 % having sub-normal and 28 % having supranormal amounts.14 Kibwage et al. reported that about 45% of drugs sampled on the Kenyan market and analysed at the Daru quality control laboratory on a routine basis were of substandard quality in terms of the content of the active ingredient.15 Shakoor et al. reported both fake and substandard drugs on Thai and Nigerian markets where 36.5% of 89 samples failed in the assay determination.6 In a study conducted by Prazuck et al. in Myanmar, it was found out that 33% (n=21) did not contain the stated dosage (one more than stated dosage and six less than stated dosage), and the highest deficit observed was 48% in two products (co-trimoxazole and benzylpenicillin).10 And thus for Malawi, the extent of the problem is within those found in other studies both in Africa and Asia.
The high failure of Co-trimoxazole in this is a worrisome development considering that it is an antibiotic of choice in most of the infections prevalent in Malawi. Of more importance to note is that in most cases Cotrimoxazole is indicated for prophylaxis for people living with HIV/AIDS, and the use of substandard medication will just aggravate the problem instead of solving it. The clinical use of medicine preparations containing less than the optimum amount of the active ingredient may be contributing to the poor treatment outcomes reported in Malawi. This may be another reason why a 2001 study by Zachariah et.al in Thyolo District of Malawi showed that co-trimoxazole resistance in E.coli among TB patients had significantly increased in a 2-year period in the district, though in that study resistance was apparently attributed to widespread availability.16 In 2006, a substandard or counterfeit pharmaceutical excipient caused more than 100 deaths in Panama.17
From the results of friability, 22.7% (5) of all tablet formulations failed the test; all of them were from public health facilities. Very few studies have been done on friability, though this may contribute to a greater loss of drugs in most African countries. The failure of the samples in the Friability indicates poor manufacturing of the tablets rather than storage conditions of the tablets. The results of friability are a worrisome development in Malawi as all of the drugs were from public health facilities. This is so because the majority of health facilities in Malawi are in rural areas where the road network is poor and characterized by bumpy and un-tarred (earth) roads, which makes the distribution of those drugs to those remote health facilities a challenge. As a result many medicines which are dispatched to those health facilities may be wasted as a result of friability or breakage, thus having a strain on drug budget expenditures.
Conclusions
This pilot study provides objective evidence to show that substandard and unregistered paracetamol and cotrimoxazole are present and being used in Malawi, possibly posing a considerable hazard to health. PMPB, together with the Ministry of Health, should develop a quality assurance system to ensure that medicines are randomly and routinely checked (post-marketing surveillance).
There is a need for a large scale study of different medicines found in Malawi to properly quantify the extent of the problem. Prospective cohort studies need to be undertaken in order to assess the effects of storage and transportation on the quality of medicines in Malawi
References
- 1.Malawi National Drug Policy; Final Draft. Lilongwe, Malawi: Ministry of Health; 2006. [Google Scholar]
- 2.Aulton ME, editor. Pharmaceutics: The Science of Dosage Form. 2nd edition. Leicester, Great Britain: Churchill Livingstone; 2002. pp. 417–420. Chapter 27. [Google Scholar]
- 3.World Health Organization, author. Report by the Secretariat; Counterfeit medical products; SIXTY-FIRST WORLD HEALTH ASSEMBLY A61/16, Provisional agenda item 1113. 2008. Apr 7, [Google Scholar]
- 4.Castro JL. Counterfeit and illegitimate medicines: a view from the Americas. WHO Drug Information. 2008;22(4) [Google Scholar]
- 5.Risha G, et al. In vitro evaluation of the quality of essential drugs on the Tanzanian market. Tropical Medicine and International Health. 2002 Aug;7(8):701–707. doi: 10.1046/j.1365-3156.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 6.Shakoor O, Taylor RB, Behrens RH. Assessment of the incidence of substandard drugs in developing countries. Tropical Medicine and International Health. 1997;2:839–845. doi: 10.1046/j.1365-3156.1997.d01-403.x. [DOI] [PubMed] [Google Scholar]
- 7.Roy J. The menace of substandard drugs. World Health Forum. 1994;15:406–407. [PubMed] [Google Scholar]
- 8.Paul NN, et al. Counterfeit anti-infective drugs. Lancet Infectious Disease. 2006;6:602–613. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 9.Sosola AG. Pharmacy, Medicines and Poisons Board Presentation as part of Pharmacy Act, Lecture Notes for Third Year Pharmacy. Malawi: Dept of Pharmacy, College of Medicine; 2008. (Deputy Registrar) [Google Scholar]
- 10.Prazuck T, Falconi I, Morineau G, et al. Quality control of antibiotics before the implementation of an STD program in Northern Myanmar. Sexually Transmitted Disease journal. 2002;29:624–627. doi: 10.1097/00007435-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 11.British Pharmacopoeia. Vol. 2. London: HMSO; 2001. [Google Scholar]
- 12. USP 32.
- 13.Gaudiano M, et al. Medicines informal market in Congo, Burundi and Angola: counterfeit and sub-standard antimalarials. Malaria Journal. 2007;6 doi: 10.1186/1475-2875-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogwal-Okeng JW, et al. Chloroquine in the Ugandan market fails quality test: a pharmacovigilance study. African Health Sciences. 2003;3(1):2–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Kibwage IO, et al. The quality work in Daru: Observations during 1983–86. East African Medical Journal. 1992;69:577–580. [PubMed] [Google Scholar]
- 16.Zachariah R, et al. Changes in Escherichia coli resistance to co-trimoxazole in tuberculosis patients and in relation to co-trimoxazole prophylaxis in Thyolo. The Royal Society Of Tropical Medicine and Hygiene. 2002;96:202–204. doi: 10.1016/s0035-9203(02)90306-8. [DOI] [PubMed] [Google Scholar]
- 17.Pincock S. WHO tries to tackle problem of counterfeit medicines in asia (News roundup) British Medical Journal. 2003 Nov;327:1126. doi: 10.1136/bmj.327.7424.1126-a. [DOI] [PMC free article] [PubMed] [Google Scholar]