Abstract
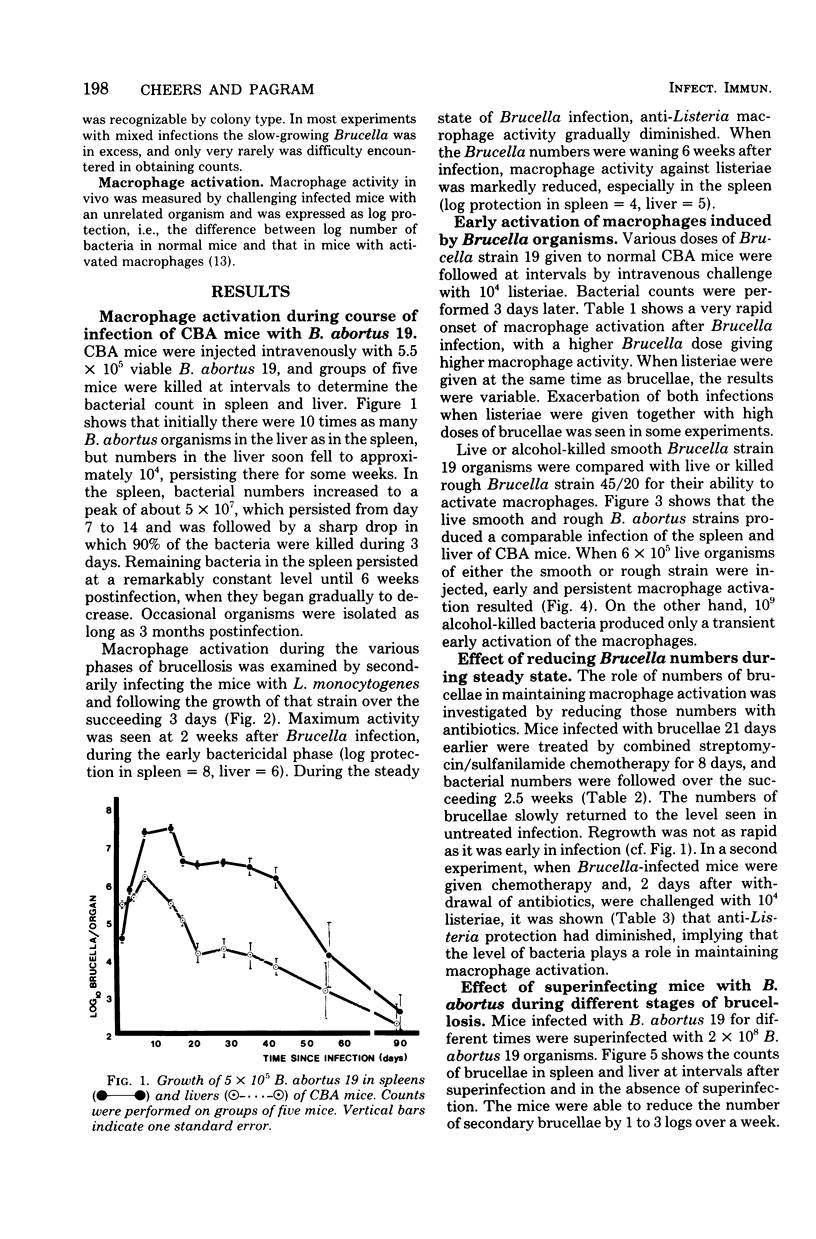
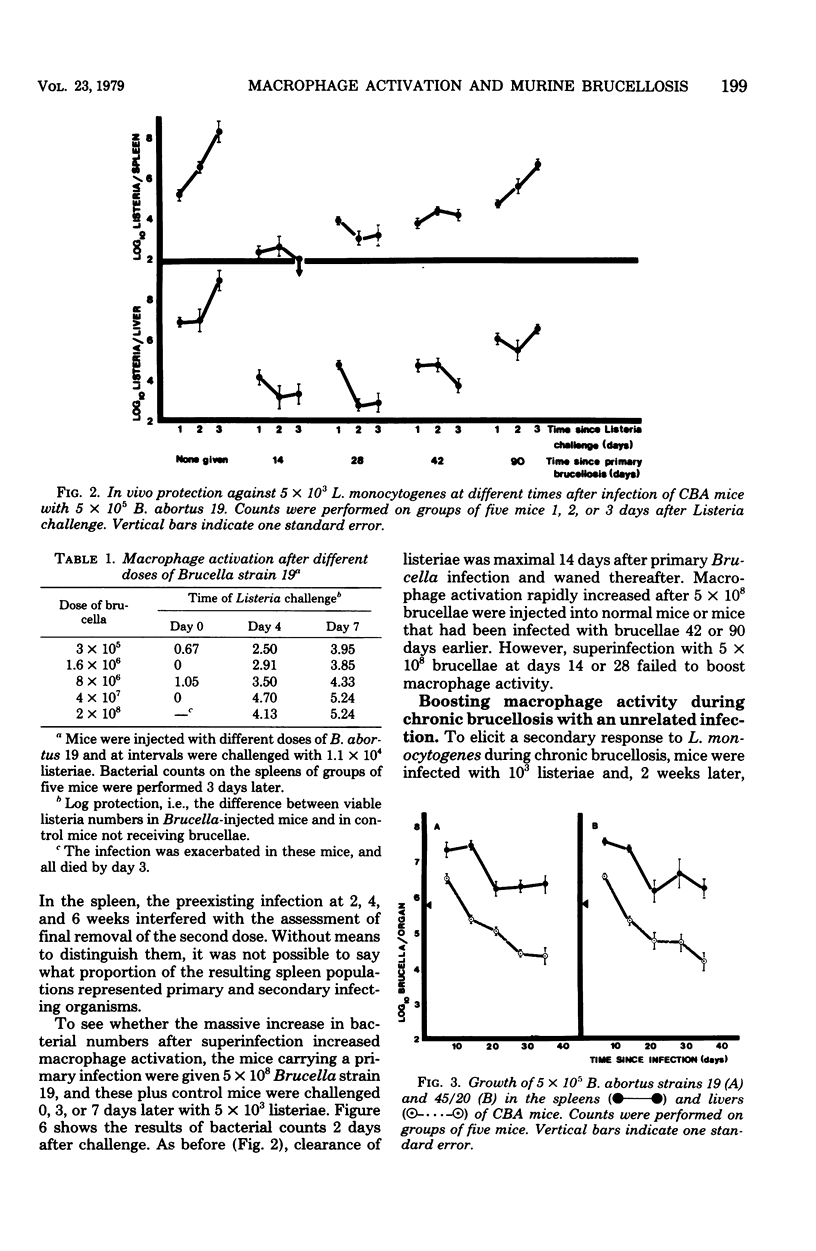
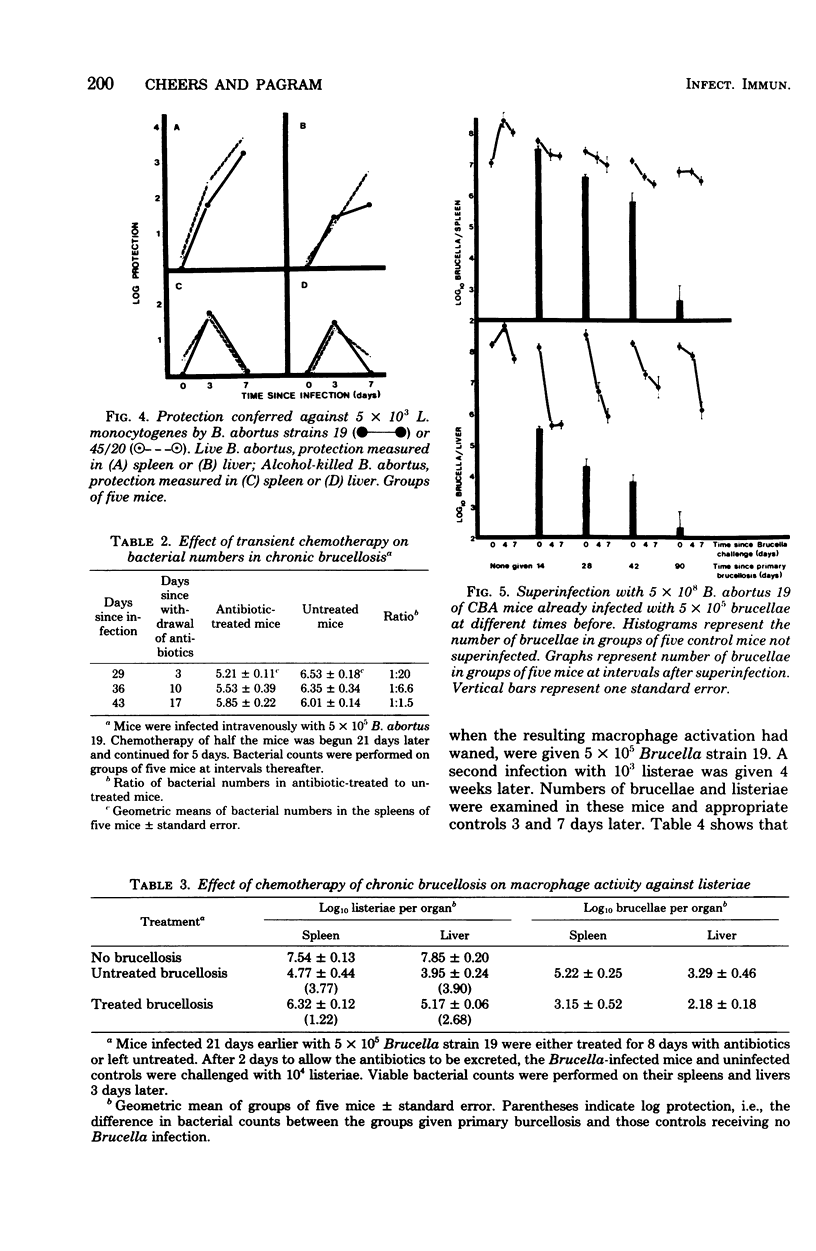
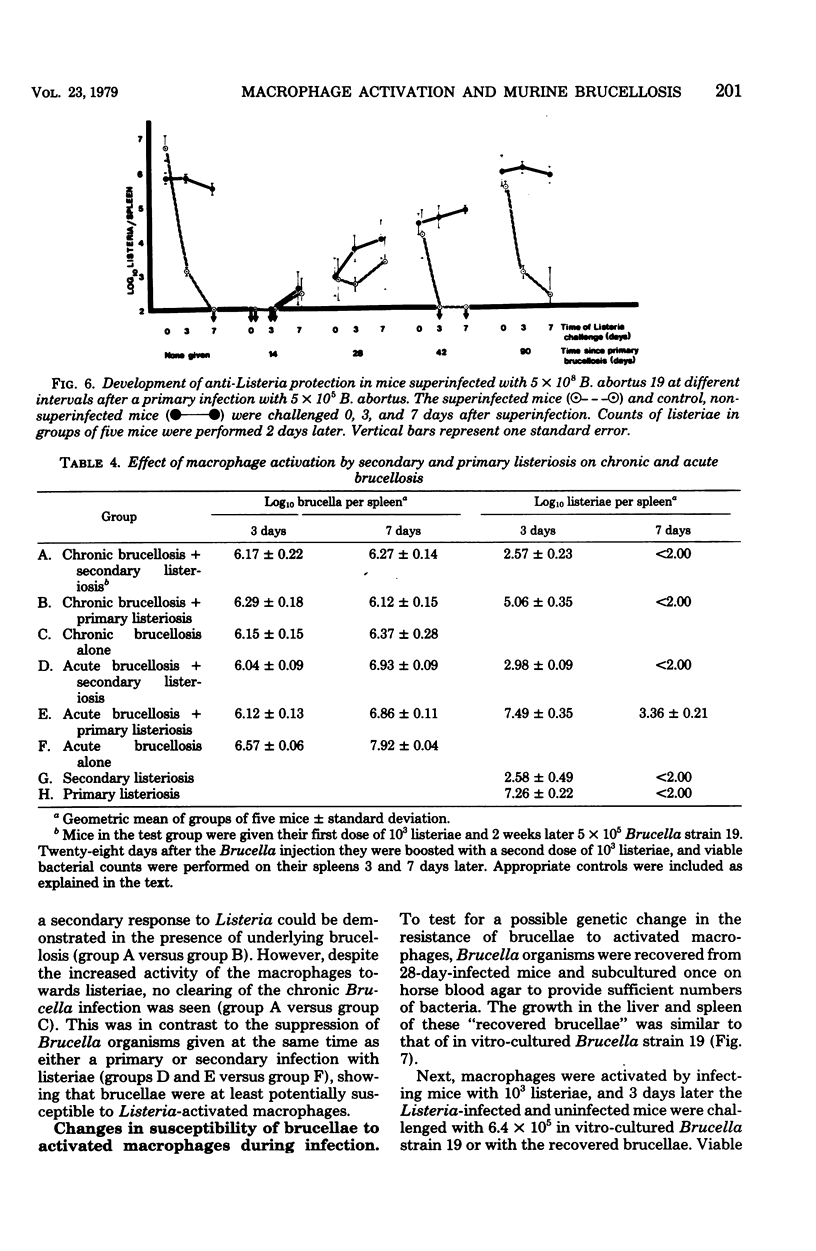
Evidence is presented that the chronicity of infection in CBA mice after injection of Brucella abortus 19 is related to a number of factors: (i) the relative resistance of B. abortus to macrophage killing, which allowed some bacteria to survive the peak of macrophage activity occurring at 14 days; (ii) the decline in macrophage activity thereafter (this decline was related in part to the presence of fewer bacteria to stimulate the bactericidal activity and also to specific, active suppressor mechanisms not identified in this study); and (iii) the insensitivity of the persistent Brucella organisms to activated macrophages. This was not due to a selection of genetically resistant bacteria, but possibly to their inaccessibility, either within "incompetent" macrophages or outside macrophages altogether.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. M., Fukui G. M., Ludwig B. J., Rosselet J. P. Increased host resistance to infection elicited by lipopolysaccharides from Brucella abortus. Proc Soc Exp Biol Med. 1969 Sep;131(4):1376–1381. doi: 10.3181/00379727-131-34111. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D. F., AFFLECK M. N. Relationship of allergy to gross lung disease and culturable bacilli in tuberculous mice. Am Rev Tuberc. 1958 Aug;78(2):226–234. doi: 10.1164/artpd.1958.78.2.226. [DOI] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The steady state in cellular immunity. I. Chemotherapy and superinfection in murine tuberculosis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):407–416. doi: 10.1038/icb.1967.39. [DOI] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The steady state in cellular immunity. II. Immunological complaisance in murine pertussis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):417–426. doi: 10.1038/icb.1967.40. [DOI] [PubMed] [Google Scholar]
- Halliburton B. L., Hinsdill R. D. Recall of acquired cellular resistance in mice by antigens from killed Brucella. Infect Immun. 1972 Jan;5(1):42–47. doi: 10.1128/iai.5.1.42-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Crandall C. A., Crandall R. B. T-dependent suppression of the primary antibody response to sheep erythrocytes in mice infected with Trichinella spiralis. Cell Immunol. 1976 Nov;27(1):102–110. doi: 10.1016/0008-8749(76)90158-1. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. S., Kirkpatrick D., Mokyr M. B., Gery I. On the mode of action of BCG. Nat New Biol. 1973 Jun 13;243(128):216–218. doi: 10.1038/newbio243216a0. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLITZKI A. L., SULITZEANU D. The use of streptomycin-resistant and nonresistant strains for the determination of the immunizing effect of living Brucella abortus vaccines in white mice. J Infect Dis. 1954 May-Jun;94(3):213–224. doi: 10.1093/infdis/94.3.213. [DOI] [PubMed] [Google Scholar]
- Pardon P., Marly J. Resistance of Brucella abortus infected mice to intravenous or intraperitoneal Brucella reinfection. Ann Immunol (Paris) 1976 Jan-Feb;127(1):57–70. [PubMed] [Google Scholar]
- Ralston D. J., Elberg S. S. Sensitization and recall of anti-Brucella immunity in Guinea pig macrophages by attenuated and virulent Brucella. Infect Immun. 1971 Feb;3(2):200–208. doi: 10.1128/iai.3.2.200-208.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Sulitzeanu D. Mechanism of immunity against brucella. Nature. 1965 Mar 13;205(976):1086–1088. doi: 10.1038/2051086a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976 Apr;13(4):1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



