Figure 2.
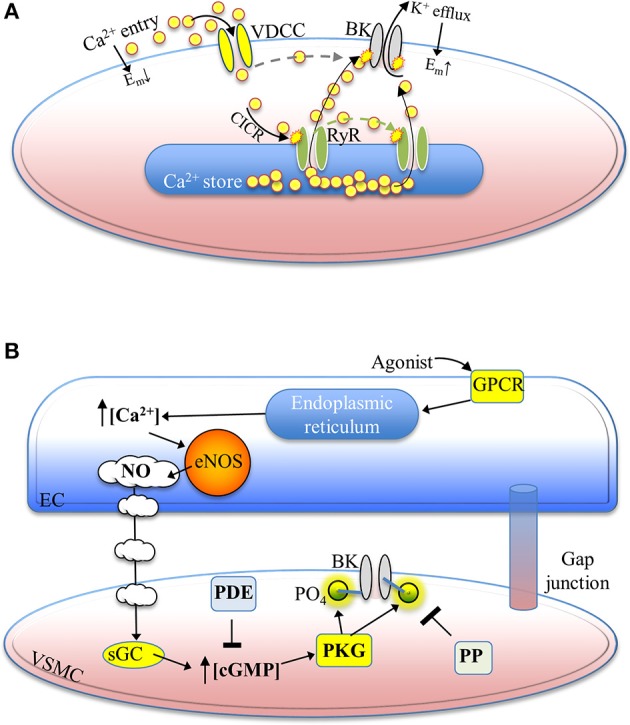
A summary of select physiological mechanisms leading to BK channel activation and reversible phosphorylation-mediated enhancement. (A) Ca2+ –dependent activation of BK channels hyperpolarizes the membrane potential. Depolarization of the membrane potential activates voltage-dependent Ca2+ channels, leading to Ca2+ entry and Ca2+-induced Ca2+ release from nearby ryanodine receptors. Released Ca2+ promotes BK channel activation, which drives the membrane potential in the negative (hyperpolarized) direction. Ca2+ influx via VDCCs may also contribute directly to BK channel activation (dotted line) as a result of the spatial proximity of these two channels within membrane nano/micro-domains. (B) Mechanisms underlying the generation of nitric oxide from an endothelial cell, with the NO/cGMP/PKG-mediated phosphorylation of a BK channel illustrated in an adjacent vascular smooth muscle cell. Nitric oxide release from endothelial cells binds to soluble guanylyl cyclase in smooth muscle cells, resulting in elevated intracellular cGMP concentrations. PKG is then activated and phosphorylates the BKα subunit. Phosphodiesterase activity lowers intracellular cGMP and protein phosphatase activity removes the regulatory phosphate from Ser/Thr residues of the BK channel protein. Abbreviations: VDCC, voltage-dependent Ca2+ channel; BK, BK channel; Em, membrane potential; CICR, Ca2+-induced Ca2+ release; RyR, ryanodine receptor; GPCR, GTP-binding protein-coupled receptor; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; EC, endothelial cell; sGC, soluble guanylyl cyclase; PDE, phosphodiesterase; PO4, phosphate group; cGMP, cyclic guanosine monophosphate; PKG, protein kinase G; PP, protein phosphatase; VSMC, vascular smooth muscle cell.
