Abstract
Background
In the UK, the use of care planning and written care plans has been proposed to improve the management of long-term conditions, yet there is limited evidence concerning their uptake and benefits.
Aim
To explore the implementation of care plans and care planning in the UK and associations with the process and outcome of care.
Design and setting
A controlled prospective cohort study among two groups of patients with long-term conditions who were similar in demographic and clinical characteristics, but who were registered with general practices varying in their implementation of care plans and care planning.
Method
Implementation of care plans and care planning in general practice was assessed using the 2009–2010 GP Patient Survey, and relationships with patient outcomes (self-management and vitality) were examined using multilevel, mixed effects linear regression modelling.
Results
The study recruited 38 practices and 2439 patients. Practices in the two groups (high and low users of written documents) were similar in structural and population characteristics. Patients in the two groups of practices were similar in demographics and baseline health. Patients did demonstrate significant differences in reported experiences of care planning, although the differences were modest. Very few patients in the cohort reported a written plan that could be confirmed. Analysis of outcomes suggested that most patients show limited change over time in vitality and self-management. Variation in the use of care plans at the practice level was very limited and not related to patient outcomes over time.
Conclusion
The use of written care plans in patients with long-term conditions is uncommon and unlikely to explain a substantive amount of variation in the process and outcome of care. More proactive efforts at implementation may be required to provide a rigorous test of the potential of care plans and care planning.
Keywords: care plans, care planning, general practice, long-term conditions, UK
INTRODUCTION
There is interest among policy makers in the use of a process of care planning and the introduction of written care plans to improve the management of long-term conditions. This interest is based in part on the Chronic Care Model,1 which suggests that long-term conditions present common challenges, with a consequent need to support patients through individualised assessment of behaviour, collaborative goal setting, self-management support, and proactive follow-up.2
Care plans and care planning
The authors have previously distinguished between care plans and care planning.3 The presence of both aspects reflects the ’gold standard’, just as the absence of both might be considered poor quality of care. It is likely that much routine care currently contains elements of the care planning process (albeit omitting the written plan), although plans without care planning may occur, for example, if the written plan is incentivised. Care plans and care planning have been implemented worldwide, including Canada4 and Australia.5,6 In the UK, care planning has been proposed for all patients with long-term conditions.7
Evidence concerning care plans and care planning
Studies in Australia have reported low levels of asthma action plans,8 with high rates in frequent users of care and those with more serious problems.9 There is national variation in the numbers of patients ‘given written instructions about how to manage their care at home’ (63% of physicians in Germany compared with 21% in the UK).10 A survey of patients found that 34–61% had agreed a plan to manage diabetes annually; however, these were not necessarily written plans.11 The authors’ previous studies suggested that 84% of patients with long-term conditions reported having a ‘care planning discussion’ within the previous 12 months and 12% reported having a written care plan.3
Most evidence on the impact of care plans and care planning relates to respiratory disorders, with reasonable consistency concerning positive effects on care use, with less evidence about benefits on quality of life.12–17 More recent trials have begun to explore the benefits of written plans in diabetes and coronary heart disease (CHD).18–22
Given limited evidence, a controlled prospective cohort study was conducted to explore the relationships between care plans, care planning, and outcomes in primary care.
How this fits in
In the UK, care planning has been proposed to improve the management of long-term conditions. However, there remains limited evidence on the implementation of care planning. This cohort study shows that the current use of written care plans is rare, that UK practices show limited variability in their implementation of care planning for long-term conditions, and that there is no relationship between levels of care planning and patient outcomes. These findings suggest that more effective methods of implementation may be required to allow the potential benefits of care planning to be demonstrated.
METHOD
Although a pragmatic randomised controlled trial of training in the delivery of care plans would have been a more rigorous method of assessing their effectiveness, there were significant practical and logistical barriers. A key issue was that care plans for patients were a policy priority at the time of the evaluation (based on wider international evidence), and randomising groups to usual care was not an available option. Thus, a controlled prospective cohort study was adopted, using natural variation in implementation of care plans and care planning to model effects on outcomes.
Practices with different levels of implementation were identified using routine data. Patients with long-term conditions within those practices were recruited using identical procedures; were assessed on a variety of measures; and were followed for 12 months. The quasi-experiment was designed to create two groups of patients who were similar in all critical respects, but who differed in their ‘exposure’ to care plans and care planning.
Practice and patient selection
To assess current implementation of care plans, the 2009–2010 GP Patient Survey (GPPS) was used.23 Questionnaires were sent in the annual survey to random samples of patients at all general practices in England (the total sample size was about 3 million), with two reminders; the overall response rate was around 40%. The focus of the version of GPPS used was on access to care and the quality of interpersonal care among all patients, but additional items related to self-reported long-term conditions. Items included those related to care planning (whether a patient with a self-reported long-term condition had discussions in the past 12 months about how best to deal with their health problem; whether, in these discussions, the doctor or nurse took notice of their views, gave them information on managing their problem, and agreed with them how best to manage their problem) and care plans (whether the responder was given a written document or care plan).
Six primary care organisations were identified as recruitment sites, representing a range of deprivation and rurality. Using the 2009–2010 GPPS data, very small practices (<1500 patients) and practices with fewer than 100 GPPS responders were excluded (to ensure stable practice-level scores), while practices where high (≥22%, n = 107) or low (≤14%, n = 98) proportions of responders with a long-term condition that reported a written document were identified. The thresholds of 14% and 22% were selected to ensure groups of roughly equal size that were well separated, with the lowest score among practices in the high group (that is, 22%) being significantly greater (P<0.05) than the highest score in the low group (that is, 14%). A propensity score method was then used to identify subgroups of 60 ‘high’ and 60 ‘low’ practices matched on a range of characteristics related to population disease burden and practice organisation (Table 1). The propensity score expresses the probability of a practice having a high score given its set of characteristics, and practices matched on these scores are typically well matched on all of the individual characteristics.24 Each practice was given a priority rating for recruitment and these ratings were updated as recruitment proceeded to maximise overall matching between the two groups.
Table 1.
Baseline characteristics of practices in the cohort, median, (interquartile range), range
| Practice characteristic | Practices with high rates of written documents (n = 17) | Practices with low rates of written documents (n = 21) | All practices (n = 38) |
|---|---|---|---|
| Percentage of patients with long-term conditions reporting written documents (GPPS)a | 27.5 (26.0–30.7) 21.5–40.3 | 10.6 (9.4–11.7) 5.6–13.4 | 12.8 (10.4–26.7) 5.6–40.3 |
| List size b | 6267 (5617–8302) 2540–16 047 | 6521 (3958–8820) 1707–13 604 | 6450 (4043–8820) 1707–16 047 |
| Practices with a General Medical Services contract,c n (%) | 8 (47) | 7 (33) | 15 (39) |
| Index of Multiple Deprivationd | 22.8 (12.2–54.0) 3.8–77.3 | 22.3 (12.3–38.4) 3.3–72.7 | 22.7 (12.2–45.4) 3.3–77.3 |
| Percentage of area population defining themselves as ‘white’e | 96.3 (90.1–98.0) 54.6–99.8 | 94.3 (86.6–97.4) 79.5–99.2 | 95.3 (90.3–98.0) 54.6–99.8 |
| Percentage of practice patients aged ≥65 yearsf | 13.5 (11.6–15.3) 5.6–20.1 | 15.2 (11.2–15.9) 5.9–20.1 | 14.1 (11.2–15.9) 5.6–20.1 |
| Percentage of practice patients who are femalef | 50.0 (49.4–50.1) 40.2–51.5 | 49.9 (47.8–50.8) 44.5–52.4 | 49.9 (48.2–50.8) 40.1–52.4 |
| Long-term condition caseloadg | 0.39 (0.35–0.42) 0.26–0.47 | 0.38 (0.35–0.44) 0.14–0.57 | 0.38 (0.35–0.43) 0.14–0.57 |
| Percentage of patients without a long-term condition reporting health to be ‘poor’ or ‘fair’a | 4.0 (3.0–7.0) 1.0–14.9 | 3.9 (3.0–5.7) 1.0–13.3 | 4.0 (3.0–6.1) 1.0–14.9 |
| Percentage of patients without a long-term condition seeing a practice nurse in last 6 monthsa | 43.6 (36.4–44.4) 27.2–56.1 | 46.5 (40.8–50.9) 31.6–59.3 | 44.4 (37.6–48.8) 27.2–59.3 |
| Percentage of patients without a long-term condition ‘very satisfied’ with care from GP surgerya | 56.0 (43.7–65.6) 33.0–74.7 | 55.7 (44.9–63.5) 34.6–78.5 | 55.9 (43.7–64.4) 33.0–78.5 |
GPPS survey 2009–2010.
Quality and Outcomes Framework 2008–2009.
General Medical Statistics 2006.
Office of National Statistics: Index of Multiple Deprivation 2007.
2001 UK census.
General Medical Statistics 2009.
Quality and Outcomes Framework 2008/9: derived by summing the registers for all chronic physical conditions in QOF and dividing by list size.
The aim was to recruit 20 practices from each group (40 in total) and a minimum of 40 patients from each, to provide 80% power (α= 0.05) to detect a small effect on patient outcomes (Cohen’s d = 0.2, intra-cluster correlation within practices of 0.05, 0.6 correlation between health status at baseline and 12 months). At each recruited practice a list of 200 patients was identified to be screened by the GP for suitability. To maximise variation in baseline health status, patients were stratified by age and number of conditions. Inclusion criteria were adults on the following disease registers: CHD; heart failure; stroke; chronic obstructive pulmonary disease; asthma; atrial fibrillation; chronic kidney disease; diabetes; and epilepsy. Anyone whom the GP deemed unsuitable, because of recent bereavement or capacity to consent, was excluded. Patients were mailed a postal survey, with two reminders, and those who returned a survey were mailed again at 6 and 12 months.
Although the GPPS was used to identify practices for the cohort, a wider range of measures was used with patients.
Measures of care plans and care planning
Care planning was assessed using validated items from the GPPS survey.23 Particular items included whether the responder had had discussions in the past 12 months with a doctor or nurse about how best to deal with their health problem; whether, in these discussions, the doctor or nurse took notice of their views, gave them information on managing their problem, and agreed with them how best to manage their problem; whether the discussions had helped to improve management of their health problem; and whether these discussions helped them better understand available support from health professionals. To assess written care plans, items from the GPPS survey were modified to include all professionals potentially involved in care planning, and other labels were included, for example, ‘personal health plan’ or ‘action plan’.
If patients replied positively to questions about care plans, they were asked additional questions by telephone interview, to gather further information and to identify other forms of written documents.
The Patient Assessment of Chronic Illness Care (PACIC) measure was also used.25,26 The content of the PACIC closely reflects elements of care planning. The scale includes 20 items in five subscales: patient activation; delivery system design and decision support; goal setting and tailoring; problem-solving and contextual counselling; and follow-up and coordination. Each item is rated on a 5-point scale from ‘almost never’ to ‘almost always’. The PACIC has good evidence of reliability and validity, but the total score was used as the items and subscales are correlated and the authors’ previous investigation of the PACIC did not support multiple subscales.25
Patient outcomes: self-management
A number of different indices relating to self-management were measured, but the primary analyses were restricted to a measure of self-management behaviour using the Summary of Diabetes Self-Care Activities scale.27 The scale asks responders to report on the number of days per week that they engaged in healthy and unhealthy behaviours. To adapt the scale for generic use, some items specific to patients with diabetes (blood sugar testing and foot care) were excluded, but five items widely recommended as healthy behaviours for people in general (eating fruit and vegetables, avoiding high-fat foods, general exercise, specific exercise, and not smoking) were kept. An item on alcohol consumption was added to these. Patients were categorised according to the number of days per week they performed healthy activities (0–3 days, 4–7 days), with >4 days a week identifying a moderate level of adherence.28 The number of healthy activities performed >4 days a week were then counted (0–5).
Patient outcomes: vitality
For the purposes of the study, a measure of vitality is reported, which the authors have previously identified as a useful generic outcome measure across conditions.29 A 5-item scale from the Medical Outcomes Study was used to assess vitality (for example, ‘did you feel worn out?’, ‘did you have enough energy?’), which was rated on a 6-point scale from ‘none of the time’ to ‘all of the time’.30,31
Sociodemographic characteristics
At baseline, sociodemographic data were gathered on sex, age, ethnicity, current work situation, and qualifications. Also included was a single-item health literacy measure,32,33 as well as the EQ-5D measure of health-related quality of life,34 and the five-item Mental Health Inventory scale, which measures general wellbeing,35,36 and can be used to assess probable depression.37
Analysis
Descriptive data are presented on the comparability of the two groups of practices and patients, on care plans and care planning (including PACIC), and on changes in self-management and vitality over time. The main analyses compared care planning, process and outcomes of care for patients in the two groups of practices. Multilevel, mixed effects linear regression modelling (the xtmixed command in Stata version 12) was employed to determine whether practice type (high or low levels of written documents) predicted PACIC scores, self-management or vitality (measured at the start of the cohort, and at 6 and 12 months follow-up), using all patients who returned the initial questionnaire (at minimum). Time-point and the interaction between practice type and time-point were included in all analyses, with the interaction removed if non-significant. Other covariates were the patient and practice-level factors used to match the practices, and patient characteristics potentially related to outcomes (Tables 1 and 2); analyses were conducted both with and without these in the model. All covariates were treated as fixed effects in the analysis and robust estimates of variance were used to allow for clustering of time-points within patients, and patients within practices.
Table 2.
Characteristics of patients
| Patient characteristic | High rate of written documents (n = 17) | Low rate of written documents (n = 21) | All practices |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 554 (51.3) | 697 (51.3) | 1251 (51.3) |
| Female | 526 (48.7) | 662 (48.7) | 1188 (48.7) |
|
| |||
| Age, years, n (%) | |||
| 18–49 | 201(18.6) | 227 (16.7) | 428 (17.6) |
| 50–64 | 331(30.7) | 419 (30.8) | 750 (30.8) |
| 65–74 | 296 (27.4) | 396 (29.1) | 692 (28.4) |
| ≥75 | 252 (23.3) | 317 (23.3) | 569 (23.3) |
|
| |||
| Health literacy, n (%) | |||
| Never | 623 (61.1) | 841 (65.3) | 1464 (63.5) |
| Rarely | 183 (17.9) | 213 (16.6) | 396 (17.2) |
| Sometimes | 127 (12.5) | 155 (12.0) | 282 (12.2) |
| Often | 30 (2.9) | 36 (2.8) | 66 (2.9) |
| Always | 56 (5.5) | 43 (3.3) | 99 (4.3) |
|
| |||
| Living situation, n (%) | |||
| Live alone | 276 (25.6) | 369 (27.2) | 645 (26.5) |
| Live with another | 804 (74.4) | 990 (72.9) | 1794 (73.6) |
|
| |||
| In paid work, n (%) | |||
| Yes | 314 (30.3) | 391 (30.2) | 705 (30.3) |
| No | 721 (69.7) | 902 (69.8) | 1623 (69.7) |
|
| |||
| Ethnicity, n (%) | |||
| White | 972 (91.3) | 1278 (95.4) | 2250 (93.6) |
| Other | 93 (8.7) | 62 (4.6) | 155 (6.4) |
|
| |||
| Count of self-reported long-term conditions, n (%) | |||
| 1 | 215 (19.9) | 286 (21.0) | 501 (20.5) |
| 2 | 295 (27.3) | 396 (29.1) | 691 (28.3) |
| 3 | 223 (20.7) | 278 (20.5) | 501 (20.5) |
| ≥4 | 347 (32.1) | 399 (29.4) | 746 (30.6) |
|
| |||
| Duration of long-term condition, n (%) | |||
| <5 years | 217 (20.6) | 299 (22.4) | 516 (21.6) |
| ≥5 years | 836 (79.4) | 1037 (77.6) | 1873 (78.4) |
|
| |||
| EQ-5D, mean (SD) | 0.69 (0.33) | 0.71 (0.31) | 0.70 (0.32) |
|
| |||
| Vitality, mean (SD) | 48.1 (22.5) | 48.5 (22.2) | 48.3 (22.4) |
|
| |||
| Wellbeing, mean (SD) | 71.7 (21.2) | 72.7 (19.7) | 72.3 (20.3) |
|
| |||
| Probable depression, n (%) | |||
| Yes | 680 (69.7) | 909 (73.1) | 1589 (71.6) |
| No | 296 (30.3) | 334 (26.9) | 630 (28.4) |
RESULTS
Descriptive data on the cohort
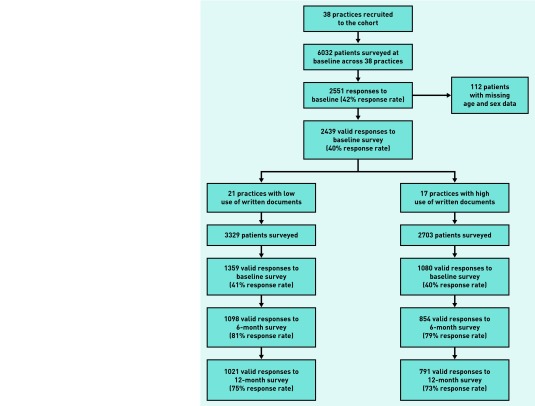
Overall recruitment to the cohort is shown in Figure 1. Thirty-eight practices were recruited, with slightly more from the low rate of written documents group (21 versus 17; Table 1). There was good differentiation between groups in rates of use of written documents from the GPPS (medians of 28% and 11%, respectively), although only one in four of the practices in the high-care group had a rate higher than 30%, and the highest reported rate of all was 40%. There was high comparability on all of the matching characteristics, apart from medical contract, with balance with respect to long-term condition caseload and the health and care reported by patients without long-term conditions was very good. A total of 2439 patients were recruited (response rates of 42% and 40% in the low and high groups, respectively). Patients showed high levels of comparability (Table 2), except that practices with a higher use of written documents tended to have more patients from ethnic minority groups.
Figure 1.
CONSORT diagram for practice and patient recruitment.
Loss to follow-up was 20% and 26% at 6 and 12 months, respectively, with very similar rates in the two practice groups (Figure 1). At 6 months, there was no effect of age, sex, education, or employment on loss to follow-up. Ethnic minority patients and those living alone were less likely to return measures. Patients reporting better health and more long-term conditions were more likely to return the questionnaire. There were no differences in baseline PACIC scores between patients responding, and those not responding, to later questionnaires, except for problem solving where responders scored slightly lower (mean 2.48 versus 2.61, P<0.05). Response rates did not differ by practice type.
Care plans and care planning in the cohort
As planned, the main differences between patients in the two groups of practices were in terms of measures of care planning for long-term conditions (Table 3). Overall, 1676 (68.7%) patients reported having had a discussion in the past 12 months about how to best deal with their health problems, with slightly higher proportions in practices defined as high users of written documents (71.9% [n = 776] versus 66.2% [n = 900]). Similar patterns were in evidence for other questions about the care planning process (Table 3). More care plans (confirmed by telephone interview) were found for patients who were registered with practices defined as high users of written documents (5% [n = 54] versus 3.2% [n = 44]), but overall rates were low, with only 98 (4%) patients having a confirmed care plan.
Table 3.
Care plans and care planning within cohort practices
| Question item | High rate of written documents (n = 17), n (%) | Low rate of written documents (n = 21), n (%) | Total (n = 38), n (%) |
|---|---|---|---|
| Care plans and care planning | |||
| Written care plan confirmed by telephone interview | 54 (5) | 44 (3.2) | 98 (4) |
| Have you had discussions in the past 12 months with a doctor or nurse about how best to deal with your health problem? | 776 (71.9) | 900 (66.2) | 1676 (68.7) |
| In these discussions, did the doctor or nurse take notice of your views about how best to deal with your health problem? | 642 (59.4) | 735 (54.1) | 1377 (56.5) |
| In these discussions, did the doctor or nurse give you information about the things you might do to deal with your health problem? | 695 (64.4) | 767 (56.4) | 1462 (59.9) |
| In these discussions, did you and the doctor or nurse agree about how best to manage your health problem? | 664 (61.5) | 728 (53.6) | 1392 (57.1) |
|
| |||
| PACIC scores (at baseline) out of 5, mean (standard deviation) | |||
| Patient activation | 2.54 (1.23) | 2.37 (1.12) | 2.45 (1.17) |
| Delivery system | 3.20 (0.97) | 3.02 (0.95) | 3.10 (1.00) |
| Goal setting | 2.31 (1.01) | 2.08 (0.91) | 2.18 (0.96) |
| Problem solving | 2.63 (1.2) | 2.43 (1.2) | 2.52 (1.9) |
| Follow-up | 2.05 (1.0) | 1.79 (0.89) | 1.91 (0.95) |
| PACIC total | 2.48 (0.92) | 2.26 (0.82) | 2.36 (0.87) |
PACIC = Patient Assessment of Chronic Illness Care measure.
Scores on the PACIC subscales were mostly below the scale mean (Table 3), with many patients reporting that they did not receive key aspects of care. There was a modest difference at baseline in mean aggregated PACIC scores between patients in the two groups of practices, largely sustained across the full 12-month follow-up period. The group difference was statistically significant both with (mean difference 0.23, 95% confidence intervals (CI) = 0.15 to 0.31, P<0.001) and without (mean difference 0.21, 95% CI = 0.12 to 0.31, P<0.001) adjustment for patient and practice characteristics (Table 4). Mean PACIC scores were significantly reduced at 6 and 12 months compared with baseline (P <0.001), but the size of the difference between the groups did not alter significantly (P >0.05).
Table 4.
Summary of multilevel regression analysis of care-planning, self-management, and vitality outcomes across all three time-points
|
Care planning (PACIC)
|
Self-management
|
Vitality
|
||||
|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | |
| Model 1 (model including covariatesa) | ||||||
| High care plan rateb,c | 0.23 (0.15 to 0.31) | <0.001 | 0.08 (–0.01 to 0.16) | 0.080 | 1.62 (0.04 to 3.20) | 0.045 |
| Time-point 2 (6 months)b,d | –0.11 (–0.15 to −0.06) | <0.001 | 0.04 (0.0 to 0.08) | 0.054 | 0.27 (–0.52 to 1.06) | 0.500 |
| Time-point 3 (12 months)b,d | –0.07 (–0.12 to −0.03) | 0.001 | 0.0 (–0.05 to 0.05) | 0.970 | –1.17 (–1.85 to −0.48) | 0.001 |
| High care plan rate x time 2e | 0.01 (–0.08 to 0.09) | 0.900 | 0.03 (–0.04 to 0.11) | 0.400 | 0.51 (–1.05 to 2.07) | 0.520 |
| High care plan rate x time 3e | –0.08 (–0.16 to 0.01) | 0.070 | –0.01 (–0.12 to 0.10) | 0.830 | 0.56 (–0.79 to 1.91) | 0.420 |
|
| ||||||
| Model 2 (model excluding covariatesa) | ||||||
| High care plan rateb,c | 0.21 (0.12 to 0.31) | <0.001 | 0.01 (–0.08 to 0.10) | 0.840 | –0.36 (–3.94 to 3.23) | 0.840 |
| Time-point 2 (6 months)b,d | –0.11 (–0.16 to −0.07) | <0.001 | 0.03 (0.0 to 0.07) | 0.070 | 0.18 (–0.48 to 0.84) | 0.600 |
| Time-point 3 (12 months)b,d | –0.09 (–0.14 to −0.05) | <0.001 | 0.01 (–0.04 to 0.06) | 0.780 | –0.95 (–1.61 to −0.28) | 0.005 |
| High care plan rate x time 2e | –0.04 (–0.12 to 0.05) | 0.380 | 0.04 (–0.04 to 0.11) | 0.340 | 0.43 (–0.87 to 1.74) | 0.527 |
| High care plan rate x time 3e | –0.08 (–0.17 to 0.01) | 0.070 | 0.0 (–0.11 to 0.10) | 0.970 | 0.55 (–0.77 to 1.87) | 0.414 |
Effects of care plans and care planning on process and outcomes of care
The overall trajectory of patients with long-term conditions was for limited change in their levels of vitality and self-management. Self-management scores did not differ between groups or between time-points either with or without covariate adjustment (Table 4, P >0.05). The unadjusted difference between practice groups in mean vitality was not significant (P = 0.84), but became significant after adjustment for practice and patient factors (P = 0.045) in favour of high users of written documents, although this was very small in size (1.6 points on a scale of 0 to 100, 95% CI = 0.04 to 3.2; a standardised effect size of 0.07). Vitality scores were significantly lower at 12 months compared with baseline (P <0.05), both with and without covariate adjustment, although again by a very small amount (−1.17 points, 95% CI = −1.85 to −0.48).
DISCUSSION
Summary
The study design was successful in creating two groups of practices that were similar apart from their implementation of care plans and care planning, and in recruiting two similar patient groups. However, reported use of written care plans was generally low, even in the ‘high care planning’ group, and the numbers of reported written plans that could be confirmed was extremely low. Thus, variation between the groups in care plans was limited, and insufficient to provide a rigorous test of any impact on outcomes.
Strengths and limitations
A total of 2439 patients were recruited from 38 practices, with over a 40% response rate. Loss to follow-up was related to important patient characteristics, but did not differ notably between the practice groups. The practices and the patients in those practices were highly similar between groups.
In the absence of a validated generic scale of healthy behaviours, the Summary of Diabetes Self-Care Activities scale was modified, keeping the items recommended for people in general. However, for some patients with particular conditions, certain behaviours may not be recommended. Scores for these patients will be less reliable, but the numbers of such patients are likely to be very small and the reduction in sensitivity to any overall difference between groups minimal.
The two groups of practices were defined using separate information from the GPPS on the prevalence of written documents. However, measurement error means that some practices may have been misclassified, reducing the ability to detect associations. Nonetheless, this selection was validated in that there were significant differences in patient reports of care planning (including PACIC). These differences, although small, are the only major differences that exist between the groups: the group difference in vitality scores was only significant after covariate control and clinically trivial in size. It could be said that the cohort was therefore successful in creating two groups of similar patients, where the major difference between groups related to the ‘interventions’ of interest, that is, care plans and care planning. It should be noted that there may be unmeasured differences or confounders between the two groups, and only a randomised controlled trial could control for these differences.
Although the cohort was not designed to assess the prevalence of care plans, the data suggest that levels are very low, and the differences between the groups on their experiences of these aspects of care were modest in size (a standardised effect size of approximately 0.25). That size of effect would be at the lower end of what one might expect of an outcome of an intervention study, whereas in the cohort this was the equivalent of the intervention itself, with the analysis designed to assess the effects of these modest differences on later outcomes. Variation in written care plans was insufficient to detect anything but a very strong association with outcomes, which seems unlikely from the existing evidence.12–17
Comparison with existing literature
The mean PACIC total was 2.4, compared with means of 2.6 in patients in US primary care,26 3.3 in primary care patients with depression in Germany,38 2.7 in patients with osteoarthritis in German primary care,39 3.0 in patients with CHD, hypertension, or diabetes in Australian general practice,40 and 3.2 in Hispanic patients with diabetes in hospital ambulatory settings in the US.41 Although there may be other variables that confound these comparisons, they do suggest that patients in the cohort perceived that they were experiencing low levels of care planning, despite high levels of overall satisfaction (Table 1).
Implications for practice and research
Research into the benefits of care plans and care planning would benefit from rigorous definition and measurement, as a detailed discussion with patients was often required to assess the presence of a plan.
Implementation of care plans and care planning in practices in the UK is sparse. This may reflect a lack of enthusiasm among professionals.4 The authors have reported qualitative research conducted alongside the cohort which has explored these issues.42
Demonstration projects in diabetes have reported higher levels of implementation in some contexts.43 Achieving higher levels of implementation to improve outcomes may require a number of methods. This might include financial or other incentives for care plans or defined care planning consultations, or implementation of care plans through other professionals outside routine primary care. It may be that care planning has a more restricted role in relation to specific issues and patient groups, such as end-of-life care,44 those with greater financial control over their care,45 and multimorbid patients with complex management regimens,46 and decisions about priorities.47
Acknowledgments
The authors would like to thank Professors Anne Rogers and Bonnie Sibbald for their contribution to the study, Mini Jarawala and Tina Audley for their assistance with the survey, and the members of the steering group for their expert advice and support.
Funding
This study was funded by the UK Department of Health Policy Research Programme (Health Reform Evaluation Programme 077/0016). This is an independent report commissioned and funded by the Policy Research Programme in the Department of Health. The views expressed are not necessarily those of the Department.
Ethical approval
Ethical approval was obtained from Northwest 3 REC – Liverpool East (REC Ref no: 10/H1002/41).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org.uk/letters
REFERENCES
- 1.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 2.Fisher EB, Brownson CA, O’Toole M, et al. Ecological approaches to self-management: the case of diabetes. Am J Public Health. 2005;95:1523–1535. doi: 10.2105/AJPH.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt J, Roland M, Paddison C, et al. Prevalence and benefits of care plans and care planning for people with long-term conditions in England. J Health Serv Res Policy. 2012;17(suppl 1):64–71. doi: 10.1258/jhsrp.2011.010172. [DOI] [PubMed] [Google Scholar]
- 4.Russell G, Thille P, Hogg W, Lemelin J. Beyond fighting fires and chasing tails? Chronic illness care plans in Ontario, Canada. Ann Fam Med. 2008;6:146–153. doi: 10.1370/afm.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwar NA, Hermiz O, Comino EJ, et al. Do multidisciplinary care plans result in better care for patients with type 2 diabetes? Aust Fam Physician. 2007;36:85–89. [PubMed] [Google Scholar]
- 6.Zwar N, Hasan I, Hermiz O. Multidisciplinary care plans and diabetes: benefits for patients with poor glycaemic control. Aust Fam Physician. 2008;37:960–962. [PubMed] [Google Scholar]
- 7.Department of Health. High quality care for all: NHS Next Stage Review (final report) London: DoH; 2008. [Google Scholar]
- 8.Sulaiman ND, Barton CA, Abramson MJ, et al. Factors associated with ownership and use of written asthma action plans in North-West Melbourne. Prim Care Respir J. 2004;13:211–217. doi: 10.1016/j.pcrj.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulaiman N, Aroni R, Thien F, et al. Written Asthma Action Plans (WAAPs) in Melbourne general practices: a sequential mixed methods study. Prim Care Respir J. 2011;20:161–169. doi: 10.4104/pcrj.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 2006;25:w555–w571. doi: 10.1377/hlthaff.25.w555. [DOI] [PubMed] [Google Scholar]
- 11.Diabetes UK, Department of Health . Care Planning in Diabetes. DoH; 2006. [Google Scholar]
- 12.Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;1:CD001117. doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- 13.Walters JA, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;5:CD005074. doi: 10.1002/14651858.CD005074.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Effing T, Monninkhof EM, van der Valk PD, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;4:CD002990. doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax. 2004;59:94–99. doi: 10.1136/thorax.2003.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell H, Gibson PG. Options for self-management education for adults with asthma. Cochrane Database Syst Rev. 2003;1:CD004107. doi: 10.1002/14651858.CD004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ring N, Malcolm C, Wyke S, et al. Promoting the use of Personal Asthma Action Plans: a systematic review. Prim Care Respir J. 2007;16:271–283. doi: 10.3132/pcrj.2007.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy AW, Cupples ME, Smith SM, et al. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ. 2009;339:b4220. doi: 10.1136/bmj.b4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant RW, Wald JS, Schnipper JL, et al. Practice-linked online personal health records for type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2008;168:1776–1782. doi: 10.1001/archinte.168.16.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiss RG, Armbruster BA, Gillard ML, McClure LA. Nurse care manager collaboration with community-based physicians providing diabetes care: a randomized controlled trial. Diabetes Educ. 2007;33:493–502. doi: 10.1177/0145721707301349. [DOI] [PubMed] [Google Scholar]
- 21.Odegard PS, Goo A, Hummel J, et al. Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Ann Pharmacother. 2005;39:433–440. doi: 10.1345/aph.1E438. [DOI] [PubMed] [Google Scholar]
- 22.Naik AD, Palmer N, Petersen NJ. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171:453–459. doi: 10.1001/archinternmed.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell J, Smith P, Nissen S, et al. The GP Patient Survey for use in primary care in the National Health Service in the UK: development and psychometric characteristics. BMC Fam Pract. 2009;10:57. doi: 10.1186/1471-2296-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statist Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Rick J, Rowe K, Hann M, et al. Psychometric properties of the Patient Assessment of Chronic Illness Care measure: acceptability, reliability and validity in United Kingdom patients with long-term conditions. BMC Health Serv Res. 2012;12:293. doi: 10.1186/1472-6963-12-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasgow RE, Wagner EH, Schaefer J, et al. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43:436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 27.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 28.Shaw BA, Gallant MP, Riley-Jacome M, Spokane LS. Assessing sources of support for diabetes self-care in urban and rural underserved communities. J Community Health. 2006;31:393–412. doi: 10.1007/s10900-006-9018-4. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy A, Reeves D, Bower P, et al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health. 2007;61:254–261. doi: 10.1136/jech.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart AL, Hays RD, Ware JE., Jr . Health perceptions, energy/fatigue, and health distress measures. In: Stewart AL, Ware JE Jr, editors. Measuring functioning and well-being: the medical outcomes study approach. Durham, NC: Duke University Press; 1992. pp. 143–172. [Google Scholar]
- 31.Lorig K, Stewart A, Ritter P, et al. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 32.Jeppesen KM, Coyle JD, Miser WF. Screening questions to predict limited health literacy: a cross-sectional study of patients with diabetes mellitus. Ann Fam Med. 2012;7:24–31. doi: 10.1370/afm.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EuroQol Group EuroQol: a new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 35.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: manual and interpretation guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 37.Harrison M, Reeves D, Harkness E, et al. A secondary analysis of the moderating effects of depression and multimorbidity on the effectiveness of a chronic disease self-management programme. Patient Educ Couns. 2012;87:67–73. doi: 10.1016/j.pec.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Gensichen J, Serras A, Paulitsch MA, et al. The Patient Assessment of Chronic Illness Care questionnaire: evaluation in patients with mental disorders in primary care. Community Ment Health J. 2011;47:447–453. doi: 10.1007/s10597-010-9340-2. [DOI] [PubMed] [Google Scholar]
- 39.Rosemann T, Laux G, Szecsenyi J, Grol R. The Chronic Care Model: congruency and predictors among primary care patients with osteoarthritis. Qual Saf Health Care. 2008;17:442–446. doi: 10.1136/qshc.2007.022822. [DOI] [PubMed] [Google Scholar]
- 40.Taggart J, Chan B, Jayasinghe UW, et al. Patients Assessment of Chronic Illness Care (PACIC) in two Australian studies: structure and utility. J Eval Clin Pract. 2011;17:215–221. doi: 10.1111/j.1365-2753.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 41.Aragones A, Schaefer EW, Stevens D, et al. Validation of the Spanish translation of the Patient Assessment of Chronic Illness Care (PACIC) survey. Prev Chronic Dis. 2008;5:A113. [PMC free article] [PubMed] [Google Scholar]
- 42.Newbould J, Burt J, Bower P, et al. Experiences of care planning in England: interviews with patients with long term conditions. BMC Fam Pract. 2012;13:71. doi: 10.1186/1471-2296-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetes UK, Department of Health, The Health Foundation, NHS. Year of care: report of findings from the pilot programme. NHS; 2011. [Google Scholar]
- 44.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson J, Baxter K, Glendinning C, et al. Personal Health Budgets: Experiences and outcomes for budget holders at nine months. Fifth Interim Report. Department of Health, 2012. http://www.dh.gov.uk/health/files/2012/06/Personal-health-budgets-evaluation-5th-Interim-Report.pdf (accessed 1 Aug 2014)
- 46.Fried TR, Tinetti ME, Iannone L. Primary care clinicians’ experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med. 2011;171:75–80. doi: 10.1001/archinternmed.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]