Abstract
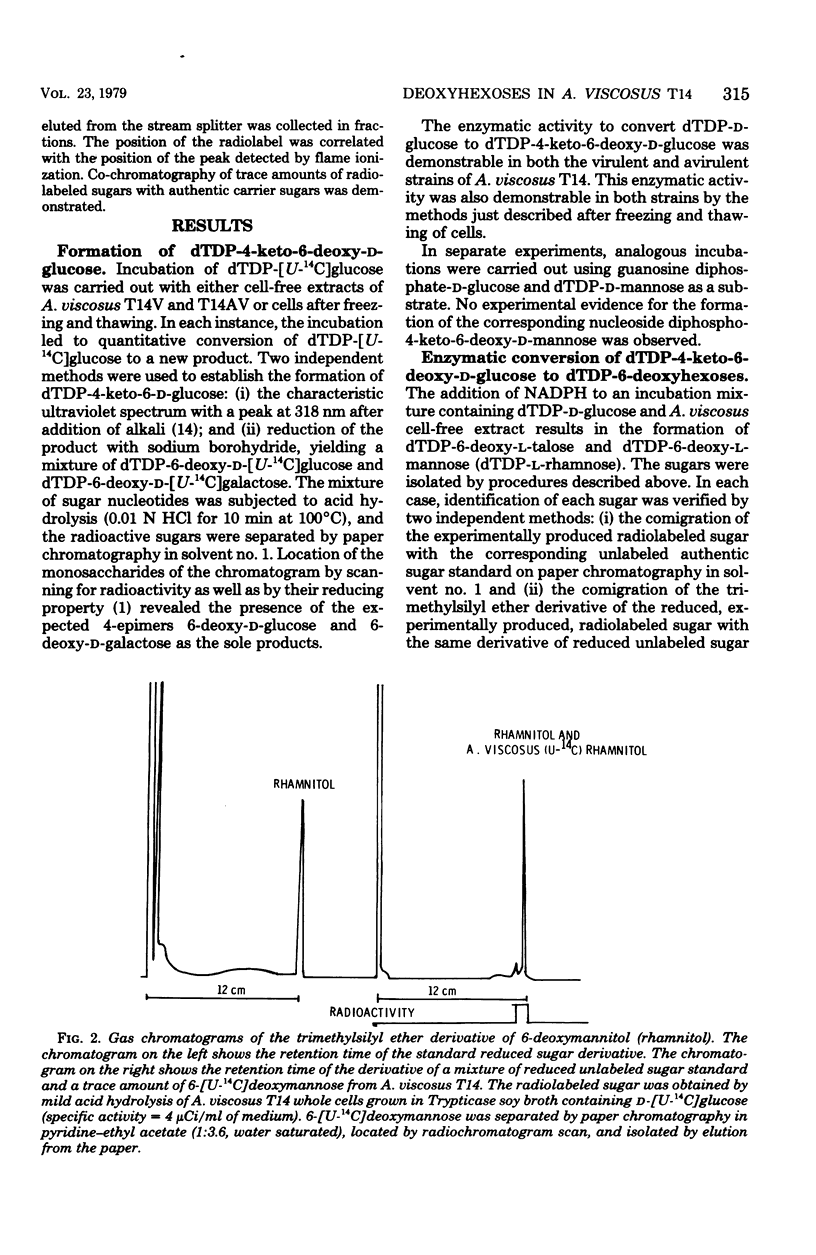
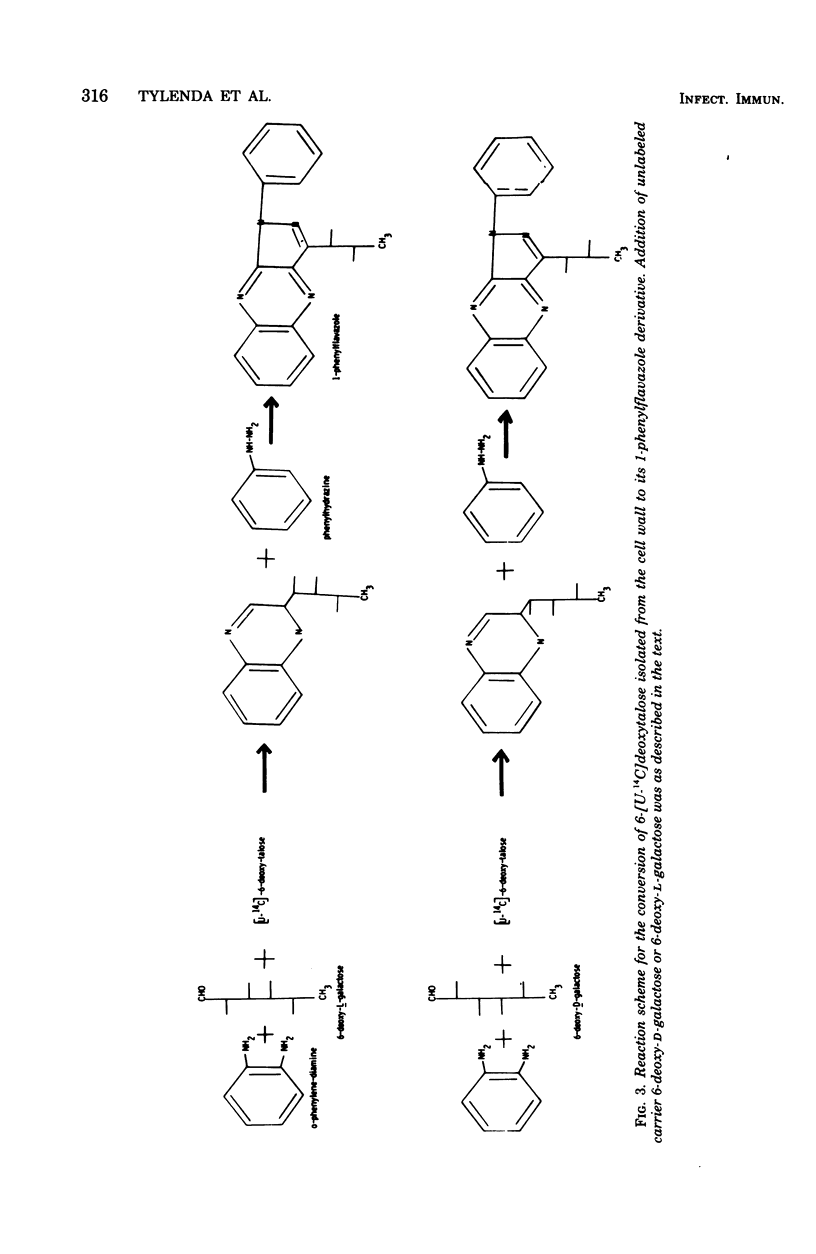
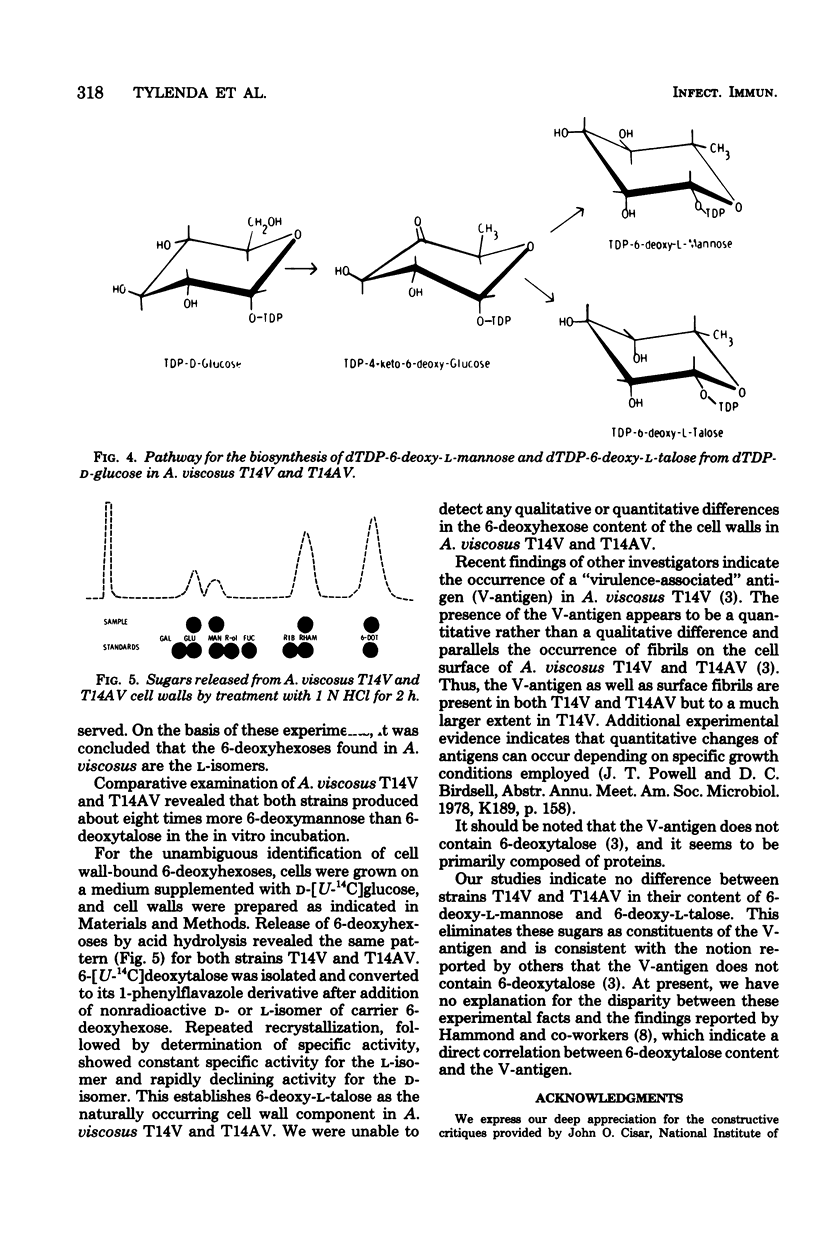
A careful examination of two strains of Actinomyces viscosus, T14V (virulent) and T14AV (avirulent), revealed no qualitative or quantitative difference in 6-deoxyhexose content of their cell surface. For a further study of the role of these sugars in cell surface-related phenomena, the stereochemical configuration of deoxyhexoses of A. viscosus T14 was established by two complementary approaches. (i) Examination of the biosynthetic pathway was found to lead to the formation of both 6-deoxy-l-talose and 6-deoxy-l-mannose and showed no differences in the ability of either bacterial strain, A. viscosus T14V or T14AV, to produce the precursors of these cell wall components. The biosynthetic pathway for 6-deoxy-l-talose and 6-deoxy-l-mannose was found to originate from deoxy-thymidine diphosphate (dTDP)-d-glucose, which in turn is converted to dTDP-4-keto-6-deoxy-d-glucose. Epimerization at carbons 3 and 5 of the hexose moiety of dTDP-4-keto-6-deoxy-d-glucose is followed by stereospecific reduction with reduced nicotinamide adenine dinucleotide phosphate to yield dTDP-6-deoxy-l-talose and dTDP-6-deoxy-l-mannose. In cell-free extracts of both A. viscosus T14 and T14AV, an identical ratio of 6-deoxy-l-talose to 6-deoxy-l-mannose of 1:8 was produced. Known precursors for the d-isomers of the same 6-deoxyhexoses such as guanosine diphosphate-d-mannose and dTDP-d-mannose were not converted by A. viscosus T14 cell-free extracts. (ii) Isolation of 6-[U-14C]deoxytalose and 6-[U-14C]deoxymannose from both strains of A. viscosus T14 was carried out by growing cells in a medium containing d-[U-14C]glucose. Again no qualitative or quantitative difference was noticeable between the two strains when 6-deoxy-hexoses were released from whole cells or purified cell walls by acid hydrolysis. Radioactive 6-[U-14C]deoxytalose isolated from the cell surface was used in an isotope dilution experiment to establish the stereochemical configuration of this 6-deoxyhexose. The radioactive sugar was mixed with unlabeled standard d- or l-6-deoxyhexose, respectively, and conversion to the corresponding 1-phenylflavazole derivative was carried out. Recrystallization to constant specific activity identified the radioactive sugar isolated from A. viscosus to be the l-isomer. A facile synthesis of the rare sugars 6-deoxy-l-talose and 6-deoxy-d-talose is reported.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
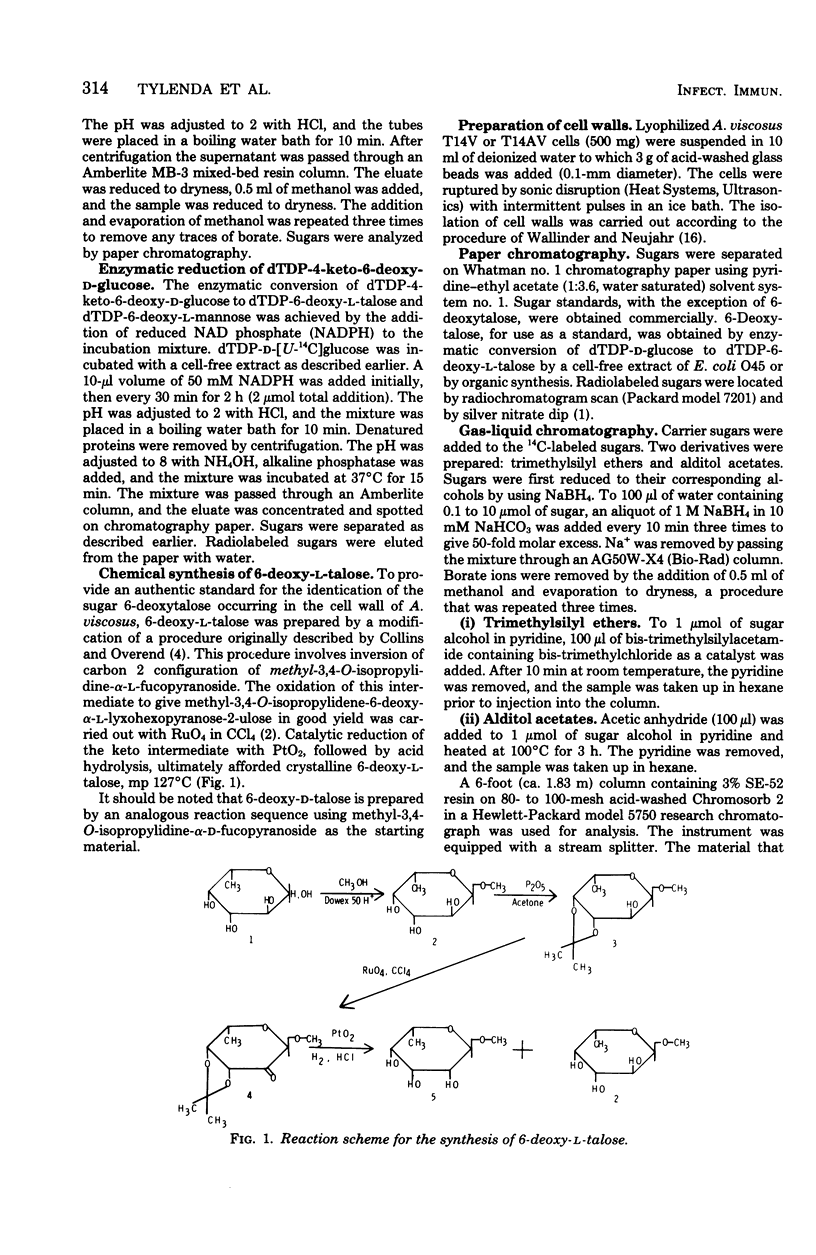
- COLLINS P. M., OVEREND W. G. A SYNTHESIS OF 6-DEOXY-L-TALOSE. J Chem Soc. 1965 Mar;65:1912–1918. doi: 10.1039/jr9650001912. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler R. W., Gabriel O. Biological mechanisms involved in the formation of deoxy sugars. VII. Biosynthesis of 6-deoxy-L-talose. J Biol Chem. 1973 Sep 10;248(17):6041–6049. [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Hickman J., Ashwell G. Isolation of a bacterial lipopolysaccharide from Xanthomonas campestris containing 3-acetamido-3,6-dideoxy-D-galactose and D-rhamnose. J Biol Chem. 1966 Mar 25;241(6):1424–1428. [PubMed] [Google Scholar]
- KORNFELD S., GLASER L. The enzymic synthesis of thymidine-linked sugars. I. Thymidine diphosphate glucose. J Biol Chem. 1961 Jun;236:1791–1794. [PubMed] [Google Scholar]
- MICHELSON A. M. SYNTHESIS OF NUCLEOTIDE ANHYDRIDES BY ANION EXCHANGE. Biochim Biophys Acta. 1964 Sep 11;91:1–13. doi: 10.1016/0926-6550(64)90164-1. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., OKAZAKIT, STROMINGER J. L., MICHELSON A. M. Thymidine diphosphate 4-keto-6-deoxy-d-glucose, an intermediate in thymidine diphosphate L-rhamnose synthesis in Escherichia coli strains. J Biol Chem. 1962 Oct;237:3014–3026. [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Wallinder I. B., Neujahr H. Y. CELL WALL AND PEPTIDOGLYCAN FROM Lactobacillus fermenti. J Bacteriol. 1971 Mar;105(3):918–926. doi: 10.1128/jb.105.3.918-926.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler N. W., Markovitz A. Guanosine diphosphate-4-keto-D-rhamnose reductase. A non-stereoselective enzyme. J Biol Chem. 1971 Oct 10;246(19):5868–5876. [PubMed] [Google Scholar]


