Abstract
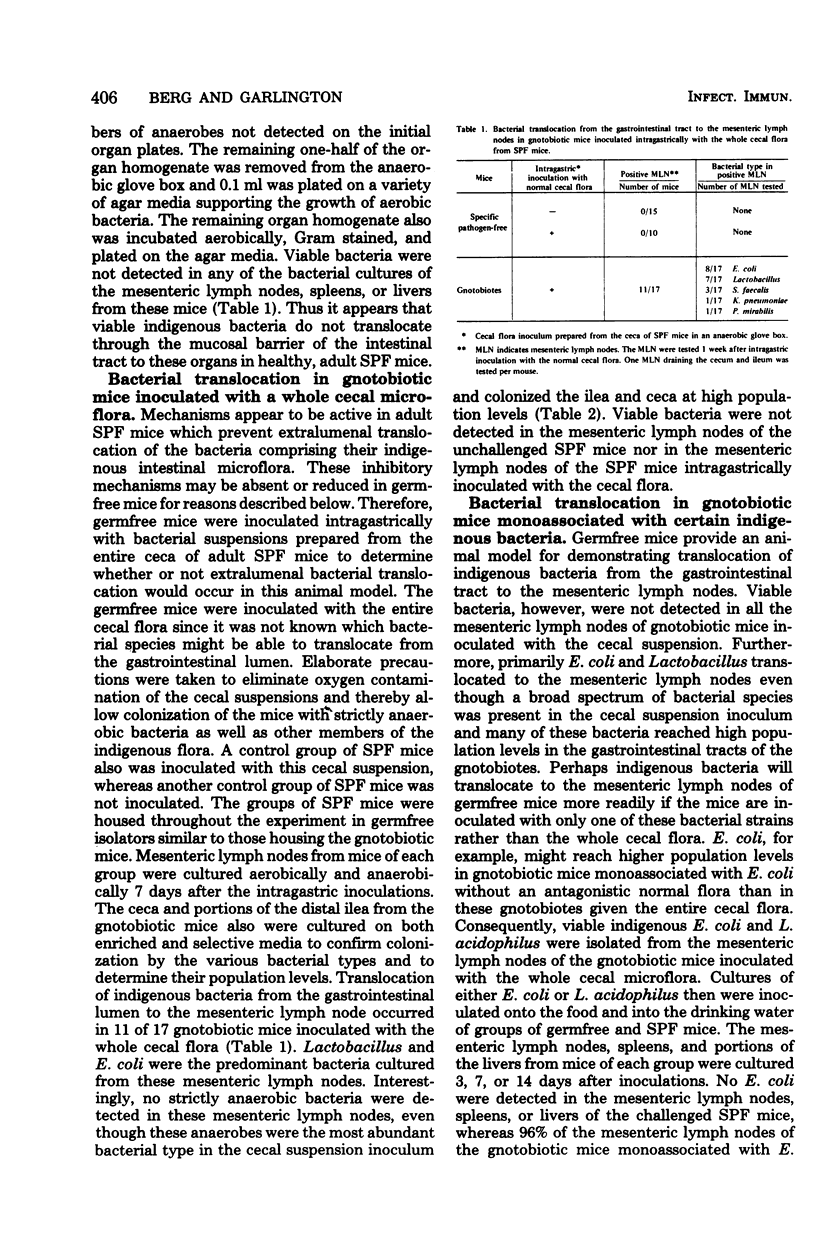
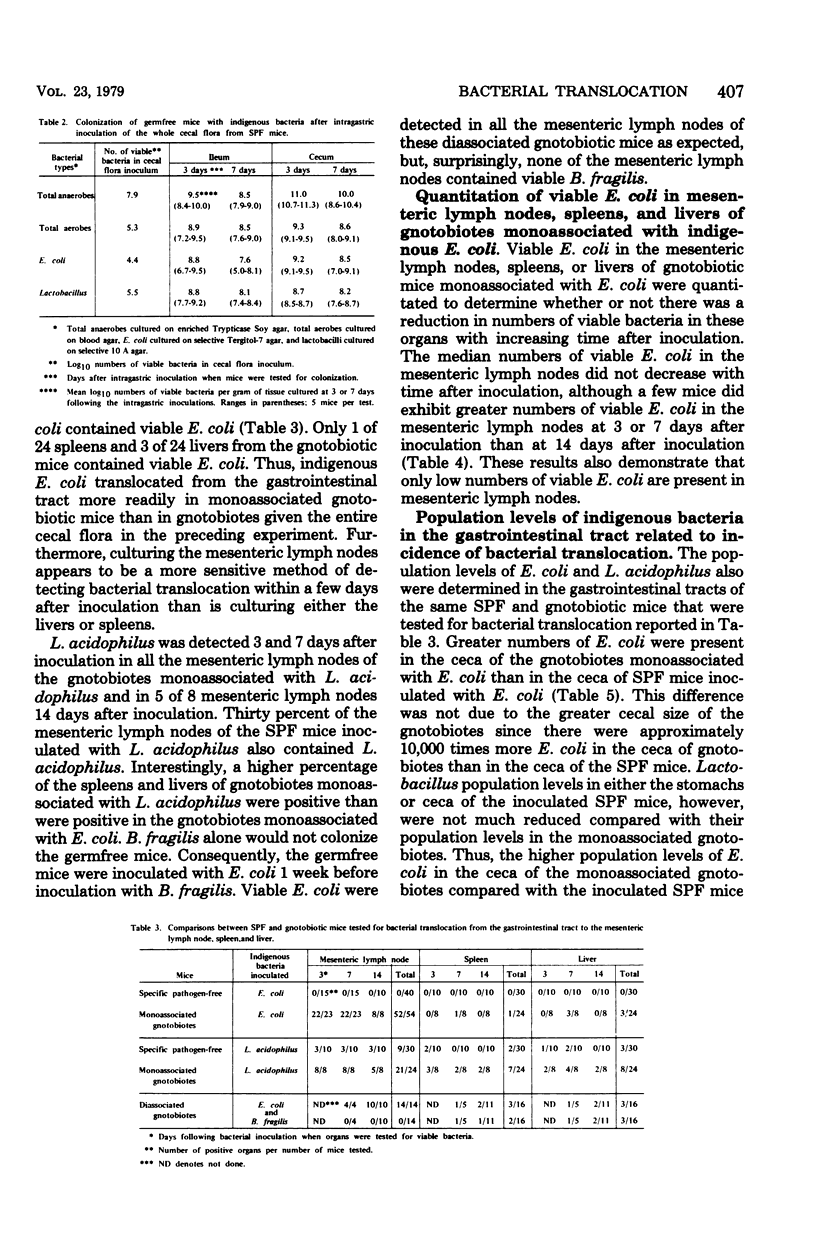
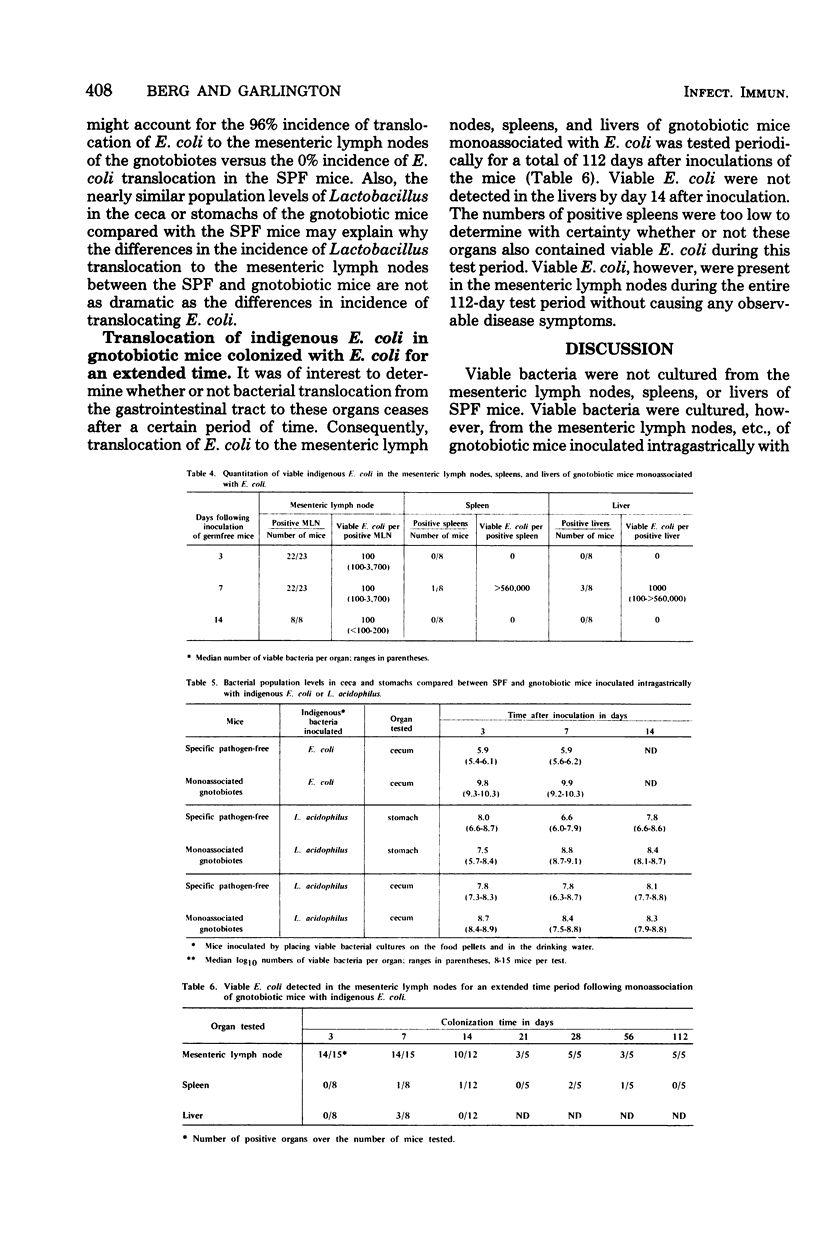
Viable bacteria were not cultured from the mesenteric lymph nodes, spleens, or livers of specific-pathogen-free (SPF) mice. Viable enteric bacteria, primarily indigenous Escherichia coli and lactobacilli, were present in the mesenteric lymph nodes of gnotobiotic mice inoculated intragastrically with the whole cecal microflora from SPF mice but not in the nodes of control SPF mice similarly inoculated. These indigenous E. coli also were cultured from the mesenteric lymph nodes of 96% of gnotobiotic mice monoassociated with E. coli but from none of the mesenteric lymph nodes of SPF mice inoculated with the E. coli. Furthermore, viable E. coli were detected in the mesenteric lymph nodes of these monoassociated gnotobiotes for as long as 112 days after inoculation. Indigenous Lactobacillus acidophilus also translocated to the mesenteric lymph nodes of gnotobiotic mice monoassociated with L. acidophilus. Apparently, there are mechanisms active in SPF mice inhibiting translocation of indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes, spleens, and livers, whereas these mechanisms are either absent or reduced in gnotobiotic mice. Indigenous E. coli maintained higher population levels in the gastrointestinal tracts of the gnotobiotes compared with their population levels in SPF mice, suggesting that high bacterial population levels might promote translocation of certain bacteria from the gastrointestinal lumen to the mesenteric lymph nodes. Gnotobiotic and SPF mice, therefore, provide experimental models for determining the nature of the mechanisms operating to confine indigenous bacteria to the gastrointestinal tract in normal, healthy animals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Lambert R., Bazin H., Heremans J. F. Interference of oral immunization with the intestinal absorption of heterologous albumin. Eur J Immunol. 1974 Oct;4(10):701–704. doi: 10.1002/eji.1830041013. [DOI] [PubMed] [Google Scholar]
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Bauer H., Paronetto F., Burns W. A., Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966 Jun 1;123(6):1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Antagonism among the normal anaerobic bacteria of the mouse gastrointestinal tract determined by immunofluorescence. Appl Environ Microbiol. 1978 Jun;35(6):1066–1073. doi: 10.1128/aem.35.6.1066-1073.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Savage D. C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975 Feb;11(2):320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Savage D. C. Immunological responses and microorganisms indigenous to the gastrointestinal tract. Am J Clin Nutr. 1972 Dec;25(12):1364–1371. doi: 10.1093/ajcn/25.12.1364. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Altemeier W. A., Bjornson H. S. Comparison of the in vitro bactericidal activity of human serum and leukocytes against bacteroides fragilis and Fusobacterium mortiferum in aerobic and anaerobic environments. Infect Immun. 1976 Sep;14(3):843–847. doi: 10.1128/iai.14.3.843-847.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Stevens W. Gut-associated lymphoepithelial tissue: bidirectional transport of tracer by specialized epithelial cells associated with lymphoid follicles. J Reticuloendothel Soc. 1977 Apr;21(4):245–254. [PubMed] [Google Scholar]
- CANADA J. C., STRONG D. H. INCIDENCE OF CLOSTRIDIUM PERFRINGENS IN THE LIVERS OF CONVENTIONAL AND GNOTOBIOTIC MICE. J Bacteriol. 1965 Jun;89:1623–1624. doi: 10.1128/jb.89.6.1623-1624.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciato D. A., Rosenblatt J. E., Goldberg L. S., Bluestone R. In vitro interaction of Bacteroides fragilis with polymorphonuclear leukocytes and serum factors. Infect Immun. 1975 Feb;11(2):337–342. doi: 10.1128/iai.11.2.337-342.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Comparative immunogenicity of heat-killed and living oral Salmonella vaccines. Infect Immun. 1972 Oct;6(4):451–458. doi: 10.1128/iai.6.4.451-458.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R., Walker W. A., Isselbacher K. J. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971 Jul;25(1):42–48. [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Cypess R. H., Swidwa D. W., Kenny J. F., Yee R. B. Influence of a metazoan infection in the mouse on enteric colonization and immune response to Escherichia coli. J Infect Dis. 1974 Nov;130(5):534–538. doi: 10.1093/infdis/130.5.534. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- GORDON L. E., RUML D., HAHNE H. J., MILLER C. P. Studies on susceptibility to infection following ionizing radiation. IV. The pathogenesis of the endogenous bacteremias in mice. J Exp Med. 1955 Oct 1;102(4):413–424. doi: 10.1084/jem.102.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale P., Hill A. The recovery of Lactobacillus sp. from the livers of healthy mice. Lab Anim. 1973 May;7(2):119–124. doi: 10.1258/002367773781008678. [DOI] [PubMed] [Google Scholar]
- Hansen S. L., Stewart B. J. Comparison of API and Minitek to Center for Disease Control methods for the biochemical characterization of anaerobes. J Clin Microbiol. 1976 Sep;4(3):227–231. doi: 10.1128/jcm.4.3.227-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F. Functional characteristics of Peyer's patch lymphoid cells. IV. Effect of antigen feeding on the frequency of antigen-specific B cells. J Immunol. 1977 Mar;118(3):992–997. [PubMed] [Google Scholar]
- Kalima T. V. Ultrastructure of the intestinal lymphatics in regard to absorption. Scand J Gastroenterol. 1973;8(3):193–196. [PubMed] [Google Scholar]
- Kenny J. F., Woleslagle D. L., Gray J. A., Michaels R. H., Pearson M. A. Enteric infection with Escherichia coli O127 in the mouse. I. Characteristics of infection and systemic and local immune responses in mice of different ages. J Infect Dis. 1970 May;121(5):528–540. doi: 10.1093/infdis/121.5.528. [DOI] [PubMed] [Google Scholar]
- Maejima K., Tajima Y. Association of gnotobiotic mice with various organisms isolated from conventional mice. Jpn J Exp Med. 1973 Aug;43(4):289–296. [PubMed] [Google Scholar]
- Nielsen M. L., Justesen T., Asnaes S., Stage J. G., Nielsen O. V. Anaerobic and aerobic bacteriological study of the liver and biliary tract in healthy laboratory animals: investigations in rabbits, guinea pigs, mice, and pigs. Dan Med Bull. 1975 Apr;22(4):159–164. [PubMed] [Google Scholar]
- Ohwaki M., Yasutake N., Yasui H., Ogura R. A comparative study on the humoral immune responses in germ-free and conventional mice. Immunology. 1977 Jan;32(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- PAYNE L. C., MARSH C. L. Absorption of gamma globulin by the small intestine. Fed Proc. 1962 Nov-Dec;21:909–912. [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBS R., COSTELLO R. ASSOCIATION OF GERMFREE MICE WITH BACTERIA ISOLATED FROM NORMAL MICE. J Exp Med. 1965 Jul 1;122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR K. B. ROLE OF IMMUNE RESPONSES IN THE GASTROINTESTINAL TRACT. Fed Proc. 1965 Jan-Feb;24:23–28. [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Grunspan R., Ganguly R. Oral immunization of mice with killed Salmonella typhimurium vaccine. Infect Immun. 1972 Jul;6(1):58–61. doi: 10.1128/iai.6.1.58-61.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules. II. Effect of parenteral immunization. J Immunol. 1973 Jul;111(1):221–226. [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules: effect of oral immunization. Science. 1972 Aug 18;177(4049):608–610. doi: 10.1126/science.177.4049.608. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk-van der Wees Colonization resistance of the digestive tract and the spread of bacteria to the lymphatic organs in mice. J Hyg (Lond) 1972 Jun;70(2):335–342. doi: 10.1017/s0022172400022385. [DOI] [PMC free article] [PubMed] [Google Scholar]


