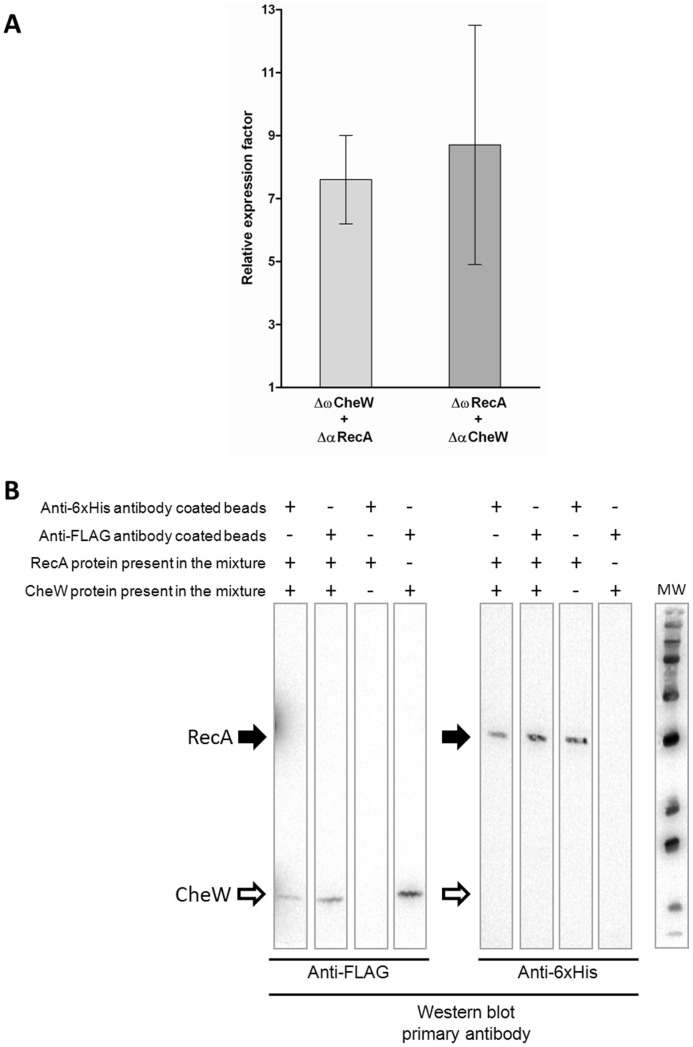
Figure 1. RecA protein directly interacts with CheW.
A) Two-hybrid assay. Measurement of the β-galactosidase activity of strains co-expressing the chimera protein pairs ΔαRecA/ΔωCheW or ΔαCheW/ΔωRecA. The results are expressed relative to those obtained with the non-interacting control strain expressing ΔαAmyA and ΔωDnaE [36]. Measurements were made 5 h after the addition of 20 nM IPTG to the culture. In each case the mean value from three independent experiments (performed in triplicate) is shown. Error bars indicate the standard deviation. B) Co-immunoprecipitation assay. Lysates prepared from cells overexpressing RecA-6×His and CheW-FLAG tagged proteins were mixed to allow the proteins to interact. Immunoprecipitation (IP) was performed by adding magnetic beads coated with either anti-6×His or anti-FLAG antibodies to the mixture and the attached proteins were recovered and separated by SDS-PAGE electrophoresis. The presence of the recombinant protein in the supernatants was assessed by western blotting (WB). As a control, co-IP assays were conducted using lysates from a ΔrecAΔcheW S. Typhimurium strain carrying an empty overexpression plasmid, thus expressing neither RecA-6×His nor CheW-FLAG proteins. The presence (+) or absence (−) of RecA, CheW, or both tagged proteins in the corresponding lysate mixture is indicated. Black and white arrows show the position of RecA-6×His and CheW-FLAG, respectively. IP indicates the antibody attached to the beads and WB the primary antibody used in western blotting. MW indicate the molecular mass marker.