Abstract
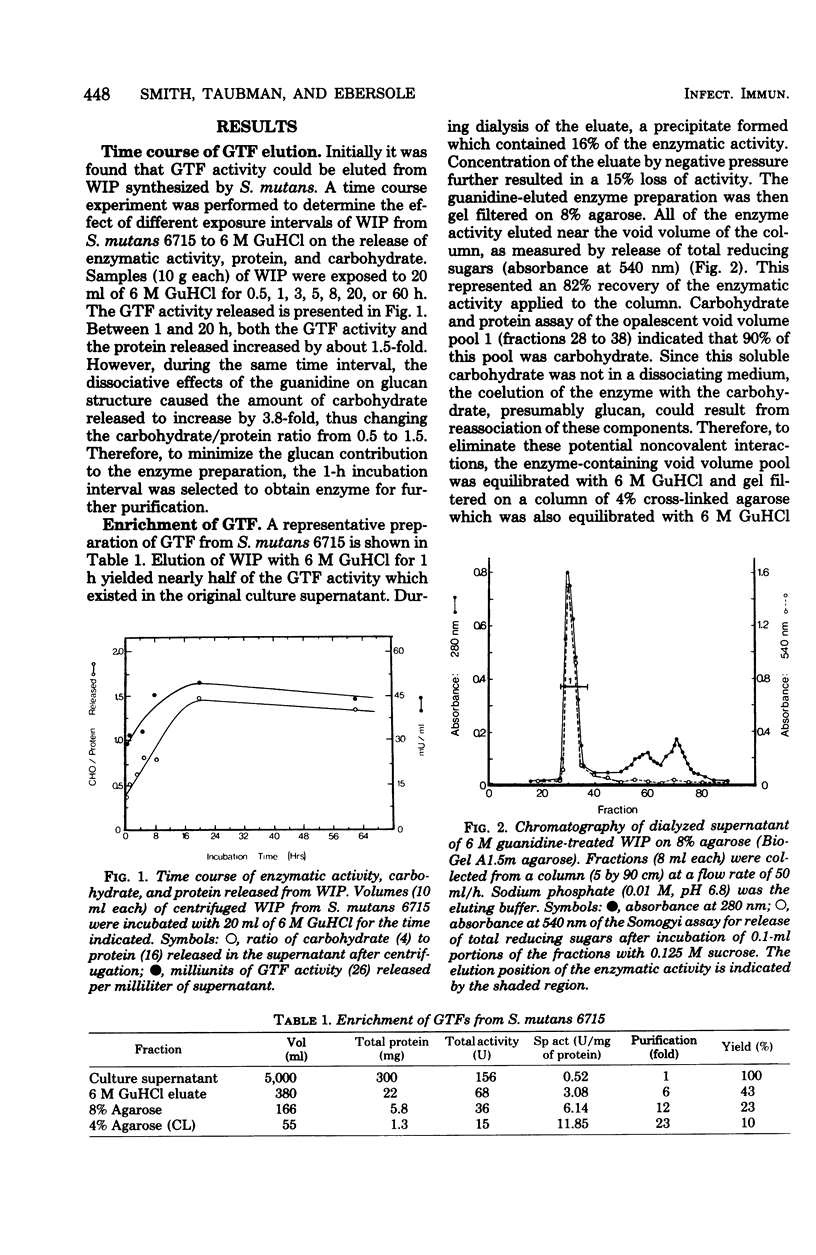
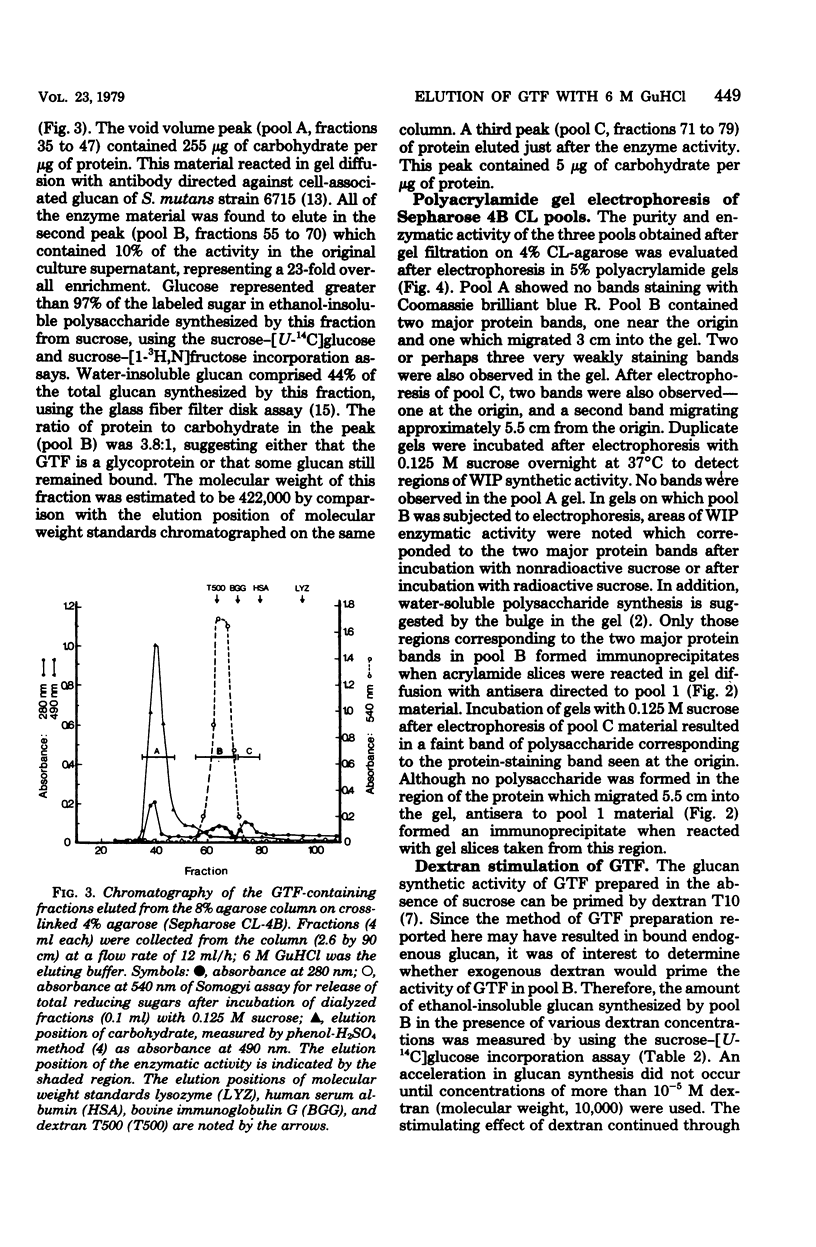
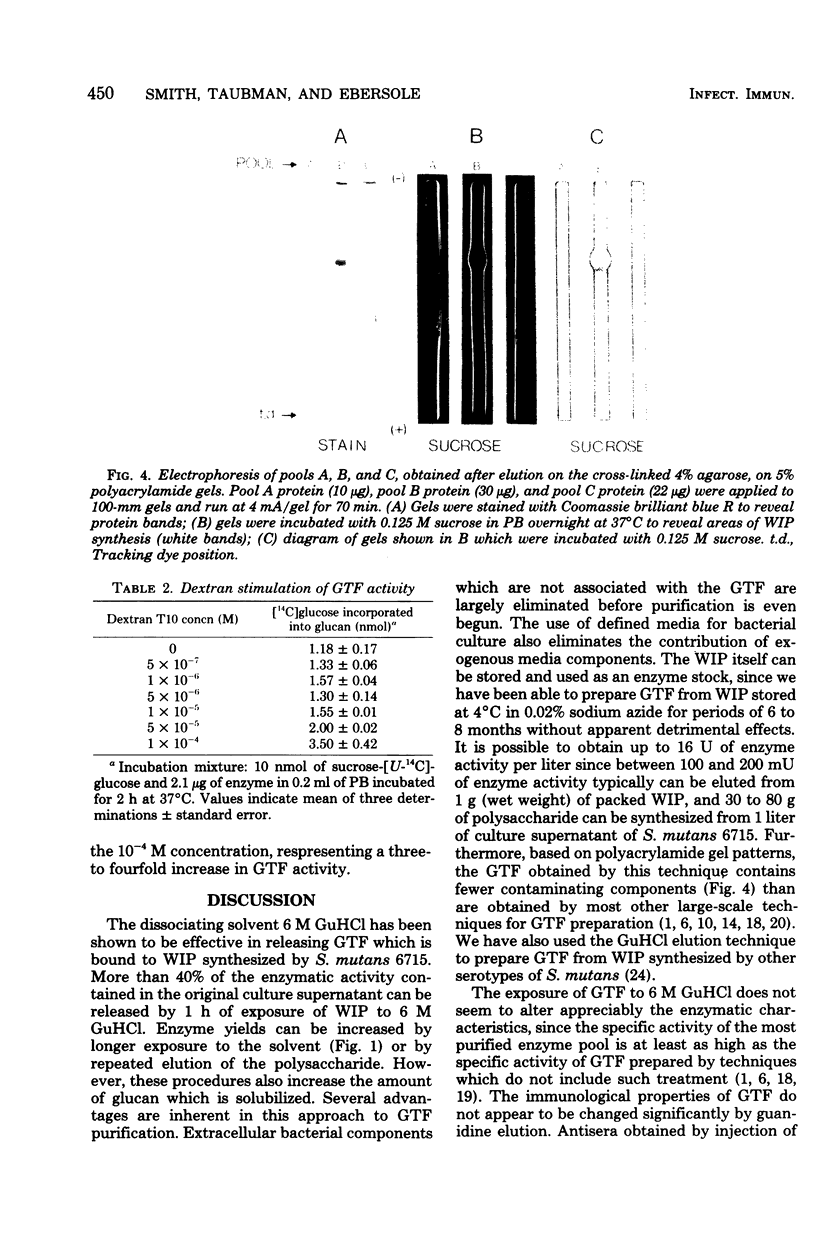
Glucosyltransferase (EC 2.4.1.5) was obtained by dissociation from water-insoluble polysaccharide in the presence of 6 M guanidine-hydrochloride. Water-insoluble polysaccharide was synthesized by cell-free culture supernatants from Streptococcus mutans strain 6715. Gel filtration of the glucosyltransferase on a column of 8% agarose in phosphate buffer, followed by filtration on a column of 4% cross-linked agarose in 6 M guanidine-hydrochloride, gave a 23-fold enrichment of the enzyme. The enriched glucosyltransferase preparation contained 22% carbohydrate and eluted at a position corresponding to a molecular weight of 422,000. Polyacrylamide gel (5%) electrophoresis of this preparation revealed two regions which stained for protein, formed water-insoluble polysaccharide in the presence of sucrose, and precipitated with antisera directed to crude glucosyltransferase preparations. The guanidine-eluted enzyme could be primed by 5 X 10(-5) M dextran T10 (molecular weight, 10,000). High-molecular-weight glucan and a possible glucan-binding protein were also obtained after the final gel filtration step (4% cross-linked agarose) in addition to glucosyltransferase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Hageage G. J., Jr, Wittenberger C. L. Multicomponent nature of the glucosyltransferase system of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C87–C96. doi: 10.1177/002203457605500330011. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Hayashi J. A., Shklair I. L., Bahn A. N. Immunization with dextransucrases and glycosidic hydrolases. J Dent Res. 1972 Mar-Apr;51(2):436–442. doi: 10.1177/00220345720510023201. [DOI] [PubMed] [Google Scholar]
- Hojo S., Huguchi M., Araya S. Glucan inhibition of diffusion in plaque. J Dent Res. 1976 Jan-Feb;55(1):169–169. doi: 10.1177/00220345760550011501. [DOI] [PubMed] [Google Scholar]
- Iacono V. J., Taubman M. A., Smith D. J., Levine M. J. Isolation and immunochemical characterization of the group-specific antigen of Streptococcus mutants 6715. Infect Immun. 1975 Jan;11(1):117–128. doi: 10.1128/iai.11.1.117-128.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Characterization of an anti-glucosyltransferase serum specific for insoluble glucan synthesis by Streptococcus mutans. Infect Immun. 1976 Feb;13(2):494–500. doi: 10.1128/iai.13.2.494-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Specific method for the purification of Streptococcus mutans dextransucrase. Infect Immun. 1977 Jun;16(3):760–765. doi: 10.1128/iai.16.3.760-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales W. R., Long L. W., Edwards J. R. Purification and characterization of a glycosyltransferase complex from the culture broth of Streptococcus mutans FA1. Carbohydr Res. 1975 Jul;42(2):325–338. doi: 10.1016/s0008-6215(00)84274-3. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Antigenic relatedness of glucosyltransferase enzymes from streptococcus mutans. Infect Immun. 1977 Jan;15(1):91–103. doi: 10.1128/iai.15.1.91-103.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in hamsters caused by homologous and heterologous serotypes of Streptococcus mutans. Infect Immun. 1978 Sep;21(3):843–851. doi: 10.1128/iai.21.3.843-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J., Ebersole J. L. Antibody binding of glucosyltransferase enzyme preparations from homologous and heterologous serotypes of S. mutans. Adv Exp Med Biol. 1978;107:317–325. doi: 10.1007/978-1-4684-3369-2_36. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]




