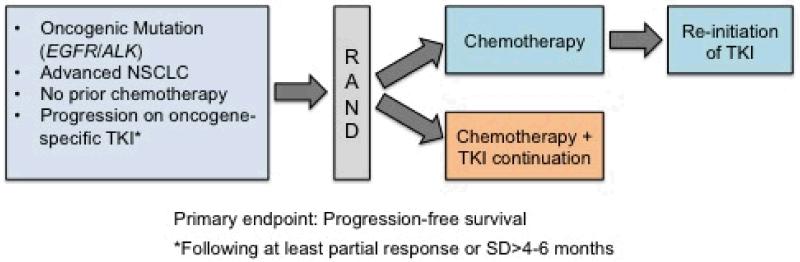
Figure 1.
Several clinical trials exploring the benefit of tyrosine kinase inhibitor (TKI) continuation with the addition of chemotherapy following progression on TKI alone are underway or proposed. The IMPRESS trial (NCT01544179; PIs, T. Mok and J.C. Soria) is currently accruing advanced NSCLC patients with EGFR activating mutations that have experienced disease progression on gefitinib and will randomize to cisplatin/pemetrexed alone or cisplatin/pemetrexed with continuation of gefitinib. A similar trial in EGFR mutation positive patients who have experienced disease progression on erlotinib will randomize patients to cisplatin or carboplatin plus pemetrexed alone or in combination with continuation of erlotinib (PI, L. Horn). This trial will allow reintroduction erlotinib in the chemotherapy arm following disease progression. The proposed SWOG 1300 (PI, D.R. Camidge) will randomize ALK positive patients with disease progression on crizotinib to either pemetrexed alone or in combination with crizotinib. Reintroduction of crizotinib following disease progression on pemetrexed will be allowed within the trial.