Left ventricular assist devices (LVADs) improve quality of life and reduce mortality of patients with heart failure.1 Although LVAD outcomes improve, unavoidable consequences of implantation remain. Given the likeliness of prior cardiac surgery, LVAD candidates for a “bridge to transplant” raise concerns regarding the number of redo sternotomies. Each sternotomy places patients at greater risk for mortality, morbidity, and resource use (eg, blood transfusions and length of stay).2 These increased risks are attributed to patients' heart failure associated with sternum-adherent dilated right ventricles (RVs).3 Direct cardiac dissection of adhesions via sternotomy can be poorly tolerated and trigger postoperative RV failure by prolonged cardiopulmonary bypass (CPB) time, bleeding, excessive transfusions, and inflammation. An alternative to redo sternotomy is a robotic endoscopic approach via thoracic chest ports. This indirect approach to the retrosternal space improves adhesion visualization, allowing more precise dissection. Reports of robotic use for redo or high-risk cases led us to assess robotic utility to reduce the invasiveness and morbidity in patients undergoing LVAD implantation.
Clinical Summary
A 49-year-old man awaiting transplant with dilated cardiomyopathy was evaluated for LVAD implantation after a decompensated period of heart failure. A miniaturized device (HVAD; HeartWare International Inc, Framingham, Mass) was implanted into the left ventricular apex via a left mini-thoracotomy incision. The da Vinci robot (Intuitive Surgical, Inc, Sunnyvale, Calif) was used to create the anastomosis of the outflow graft with the ascending aorta. With the patient supine, the right femoral vessels were cannulated for CPB. A small left anterior thoracotomy exposed the cardiac apex, localized via preoperative chest computed tomography imaging, and the inflow sewing ring was sutured into place. The pump was positioned within the left thorax, and the drive line was tunneled subcutaneously over the lower left ribs.
With the right lung isolated, 3 small robotic ports were placed in the right chest via the second (left robotic arm), third (camera and working port), and fifth (right robotic arm) intercostal spaces in the anterior axillary line Figure 1). Robotic assistance was used to pass the outflow graft through a mediastinal tunnel created anterior to the RV into the right chest for anastomosis with the aorta (Figure 2). Direct visualization allowed for accurate measurement and placement along the diaphragm (conventional placement of outflow) and prevented kinking.
Figure 1.
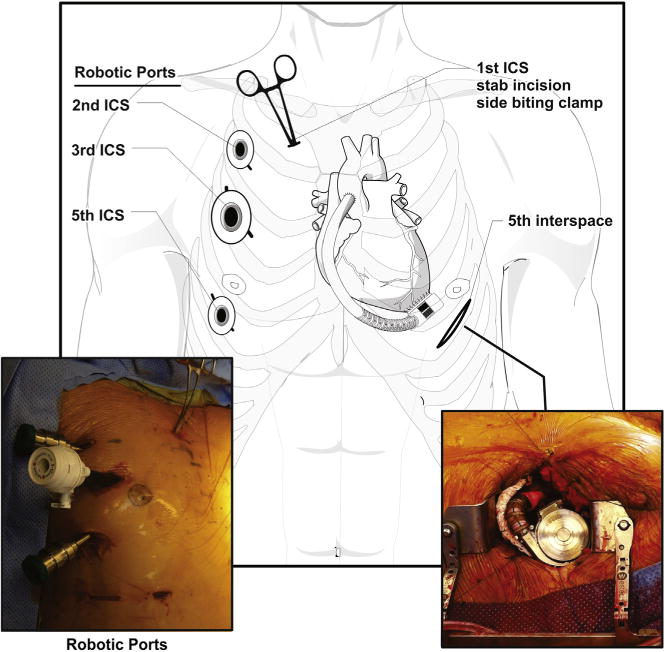
Placement of robotic port sites in intercostal spaces (ICS) and incisions with depiction of the HVAD (HeartWare International Inc, Framingham, Mass) after implantation.
Figure 2.
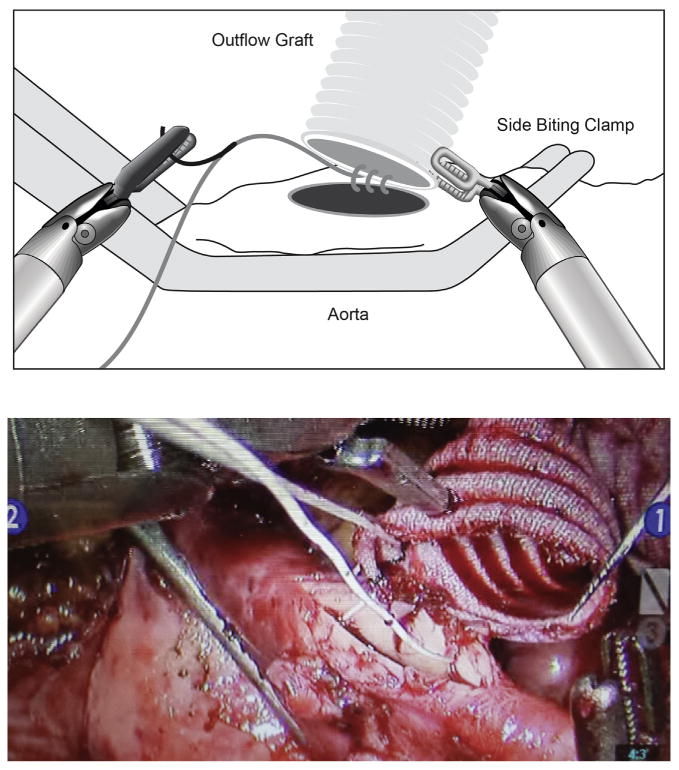
Total endoscopic anastomosis of outflow graft to aorta using the da Vinci robot (Intuitive Surgictal, Inc, Sunnyvale, Calif).
A side-biting clamp was placed onto the ascending aorta via a stab incision in the first intercostal space, right of midline with direct visualization to avoid injuring the right internal thoracic artery. CPB was initiated after appropriate activated clotting time–guided heparinization; an apical core was removed from the left ventricle, and the HVAD was secured into position. The outflow cannula was anastomosed to the aorta with 5.0 running polytetrafluoroethylene (Gore-Tex; WL Gore & Associates Inc, Flagstaff, Ariz) suture performed initially by hand in the first few patients and then totally endoscopically using robotic instruments with and without a 2-cm right anterior thoracotomy. Once the device was placed, flow through the device was initiated, and an angiocatheter was placed into the outflow graft to de-air through the third intercostal space. The device was covered with a polytetrafluoroethylene (Gore-Tex) mesh to minimize lung adhesions.
For 7 cases, bypass times ranged from 68 to 136 minutes. Intraoperative blood product use ranged from 0 to 3 units of red blood cells, 2 to 3 units of fresh frozen plasma, and 1 to 2 pooled platelet units. Preoperatively, all patients had at least moderate RV dysfunction determined by cardiac magnetic resonance imaging (RV ejection fraction range, 20%-35%). Yet, postoperative RV failure did not develop in any of the patients. Four patients were extubated between 12 and 24 hours after surgery. The other 3 patients were extubated on postoperative days 2, 5, and 7.
Discussion
As LVAD support for patients with heart failure becomes increasingly popular, concern for redo sternotomies increases.4 Further, reoperative sternotomy at the time of subsequent heart transplantation has been associated with decreased short- and long-term survival. To avoid redo sternotomy, new methods of LVAD implantation must be explored, particularly as new-generation devices become smaller and more conducive to minimally invasive implantation. Our approach improves on existing less-invasive approaches for LVAD implantation because robotic technology provides optimal visualization for RV dissection and reduced risk of kinking the outflow graft.5 Performing the anastomosis of the graft to the ascending aorta through right chest ports further decreases mediastinal dissection. This may improve outcomes in these patients at the time of transplantation. Furthermore, the robotic ports discussed are suitable for concomitant procedures usually performed through the right chest.
Conclusions
Refinements in surgical technique, team development and training methods, and patient selection and management will enable robotic technology to play an important role in LVAD implantation.
Acknowledgments
The authors thank Katherine Stavoe and Kitsie Penick for assistance with article preparation and Rita Ellsworth for developing the figures. Dr Poston is supported by grants from Intuitive Surgical Inc and the National Institutes of Health (RO1 HL084080).
Footnotes
Disclosures: All other authors have nothing to disclose with regard to commercial support.
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.George TJ, Beaty CA, Ewald GA, Russell SD, Shah AS, Conte JV, et al. Reoperative sternotomy is associated with increased mortality after heart transplantation. Ann Thorac Surg. 2012;94:2025–32. doi: 10.1016/j.athoracsur.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park CB, Suri RM, Burkhart HM, Greason KL, Dearani JA, Schaff HV, et al. Identifying patients at particular risk of injury during repeat sternotomy: analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg. 2010;140:1028–35. doi: 10.1016/j.jtcvs.2010.07.086. [DOI] [PubMed] [Google Scholar]
- 4.Kamdar F, John R, Eckman P, Colvin-Adams M, Shumway SJ, Liao K. Postcardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2013;145:575–81. doi: 10.1016/j.jtcvs.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 5.Cheung A, Lamarche Y, Kaan A, Munt B, Doyle A, Bashir J, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg. 2011;91:1294–6. doi: 10.1016/j.athoracsur.2010.08.031. [DOI] [PubMed] [Google Scholar]


