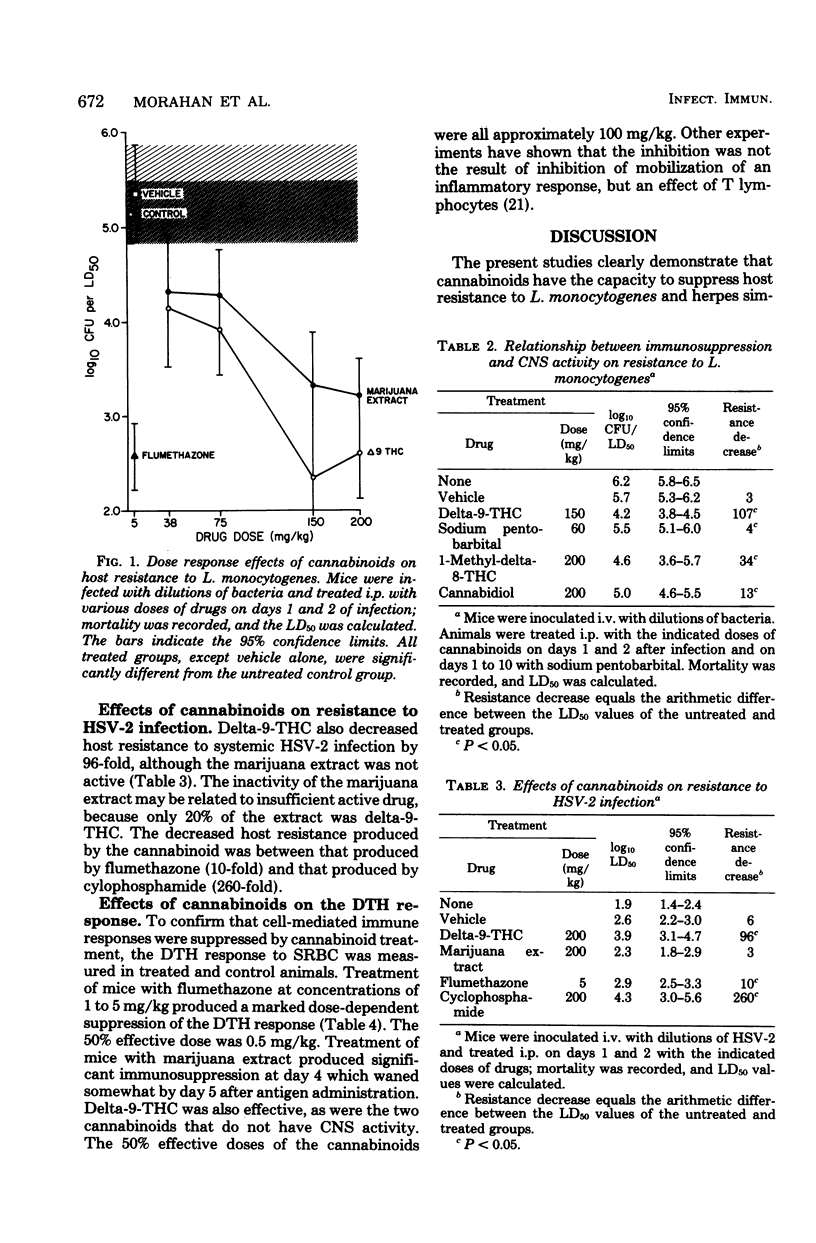
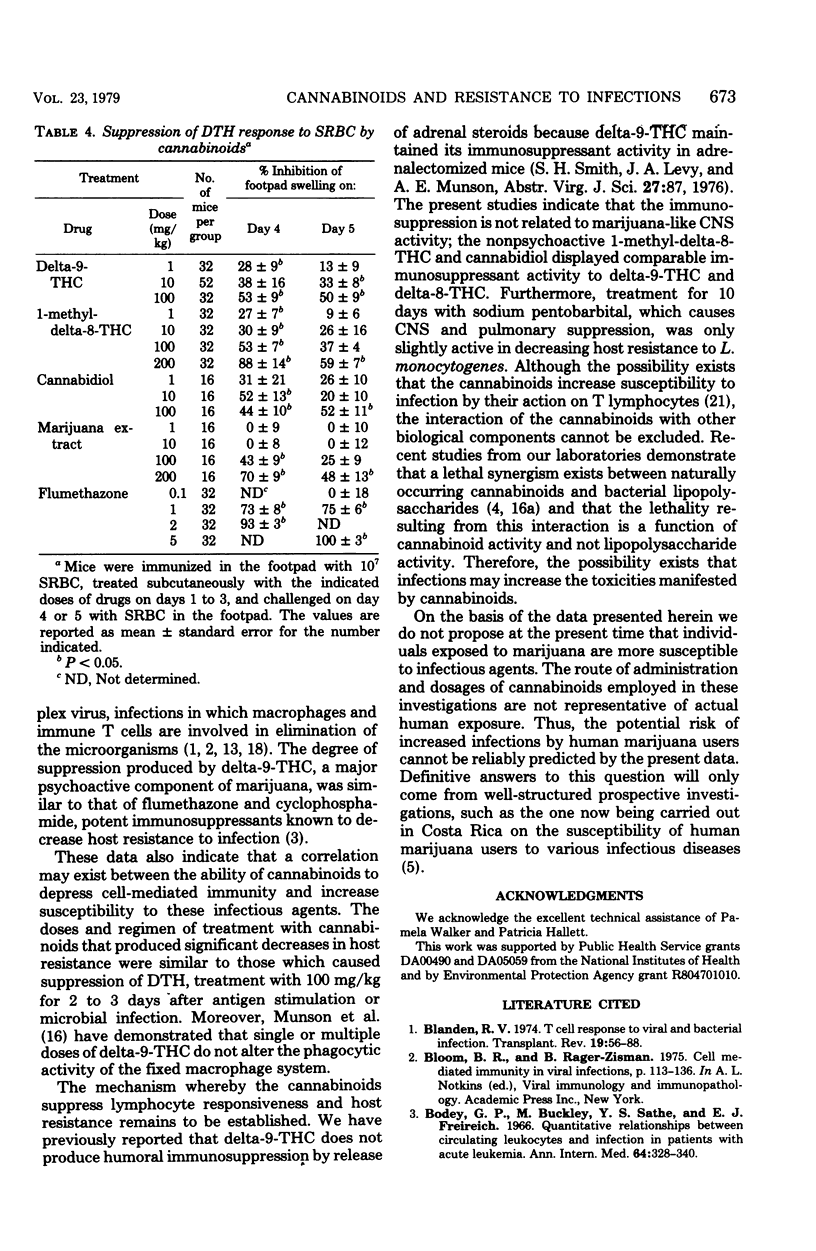
Abstract
Previous investigations from our laboratories have demonstrated that cannabinoids possess immunosuppressive properties. The present studies were designed to determine whether these agents decrease host resistance to infections with Listeria monocytogenes and herpes simplex virus type 2. Host resistance was measured by changes in the 50% lethal dose of the pathogen in cannabinoid-treated and control mice. The effect of cannabinoids on resistance to L. monocytogens was dose dependent. Delta-9-tetrhydrocannabinol at doses of 38, 75, and 150 mg/kg suppressed resistance to infection by 10-, 17-, and 657-fold, respectively. Marijuana extract was less active but significantly reduced resistance to L. moncytogenes at all tested doses. Resistance to systemic herpes simplex virus type 2 infection was decreased 96-fold by delta-9-tetrahydrocannabinol, although marijuana extract was inactive. The doses and regimen of treatment with cannabinoids that produced significant decreases in host resistance were similar to those which caused suppression of delayed-type hypersensitivity to sheep erythrocytes. The possible mechanisms and public health aspects of the decreased host resistance produced by marijuana extract and its cannabinoids are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Buckley M., Sathe Y. S., Freireich E. J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966 Feb;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Bradley S. G., Munson A. E., Dewey W. L., Harris L. S. Enhanced susceptibility of mice to combinations of delta 9-tetrahydrocannabinol and live or killed gram-negative bacteria. Infect Immun. 1977 Aug;17(2):325–329. doi: 10.1128/iai.17.2.325-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman P., Khurana R. Marijuana and T lymphocyte rosettes. Clin Pharmacol Ther. 1976 Mar;19(3):310–317. doi: 10.1002/cpt1976193310. [DOI] [PubMed] [Google Scholar]
- Esber H. J., Menninger F. F., Jr, Bogden A. E., Mason M. M. Immunological deficiency associated with cigarette smoke inhalation by mice. Primary and secondary hemagglutinin response. Arch Environ Health. 1973 Aug;27(2):99–104. doi: 10.1080/00039896.1973.10666328. [DOI] [PubMed] [Google Scholar]
- Gupta S., Grieco M. H., Cushman P., Jr Impairment of rosette-forming T lymphocytes in chronic marihuana smokers. N Engl J Med. 1974 Oct 24;291(17):874–877. doi: 10.1056/NEJM197410242911704. [DOI] [PubMed] [Google Scholar]
- Klykken P. C., Smith S. H., Levy J. A., Razdan R., Munson A. E. Immunosuppressive effects of 8,9-epoxyhexahydrocannabinol (EHHC). J Pharmacol Exp Ther. 1977 Jun;201(3):573–579. [PubMed] [Google Scholar]
- Lefkowitz S. S., Chiang C. Y. Effects of delta-9-tetrahydrocannabinol on mouse spleens. Res Commun Chem Pathol Pharmacol. 1975 Aug;11(4):659–662. [PubMed] [Google Scholar]
- Mann P. E., Cohen A. B., Finley T. N., Ladman A. J. Alveolar macrophages. Structural and functional differences between nonsmokers and smokers of marijuana and tobacco. Lab Invest. 1971 Aug;25(2):111–120. [PubMed] [Google Scholar]
- Morahan P. S., Breinig M. C., McGeorge M. B. Immune responses to vaginal or systemic infection of BALB/c mice with herpes simplex virus type 2. J Immunol. 1977 Dec;119(6):2030–2036. [PubMed] [Google Scholar]
- Munson A. E., Sanders V. M., Bradley S. G., Loveless S. E., Harris L. S. Lethal interaction of bacterial lipopolysaccharide and naturally occurring cannabinoids. J Reticuloendothel Soc. 1978 Dec;24(6):647–655. [PubMed] [Google Scholar]
- Nahas G. G., Suciu-Foca N., Armand J. P., Morishima A. Inhibition of cellular mediated immunity in marihuana smokers. Science. 1974 Feb 1;183(4123):419–420. doi: 10.1126/science.183.4123.419. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Paranjpe M. S., Boone C. W. Delayed hypersensitivity to simian virus 40 tumor cells in BALB-c mice demonstrated by a radioisotopic foot-pad assay. J Natl Cancer Inst. 1972 Feb;48(2):563–566. [PubMed] [Google Scholar]
- Schwartzfarb L., Needle M., Chavez-Chase M. Dose-related inhibition of leukocyte migration by marihuana and delta-9-tetrahydrocannabinol (THC) in vitro. J Clin Pharmacol. 1974 Jan;14(1):35–41. doi: 10.1002/j.1552-4604.1974.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Harris L. S., Uwaydah I. M., Munson A. E. Structure-activity relationships of natural and synthetic cannabinoids in suppression of humoral and cell-mediated immunity. J Pharmacol Exp Ther. 1978 Oct;207(1):165–170. [PubMed] [Google Scholar]
- Wildes J. W., Martin N. H., Pitt C. G., Wall M. E. The synthesis of (-)-delta-9(11)-trans-tetrahydrocannabinol. J Org Chem. 1971 Mar 12;36(5):721–723. doi: 10.1021/jo00804a024. [DOI] [PubMed] [Google Scholar]


