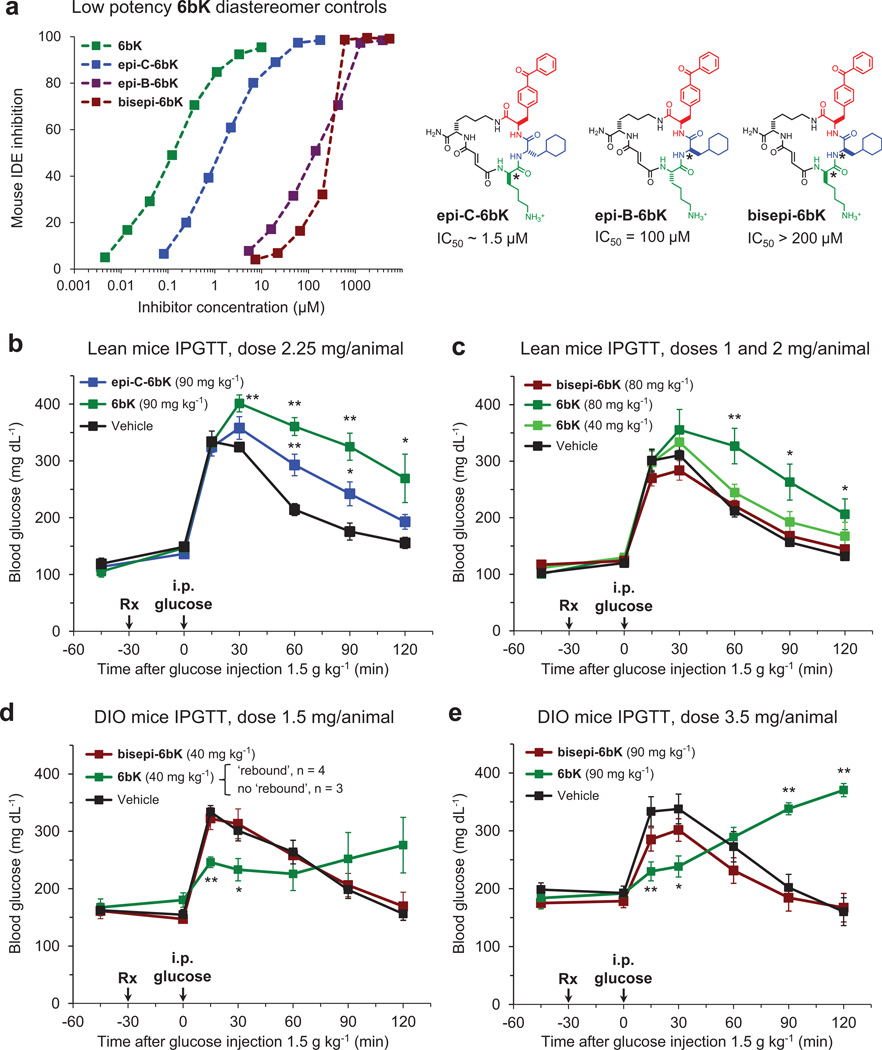
Extended Data Figure 7. Low-potency diastereomers of 6bK used to determine effective dose range and confirm on-target IDE inhibition effects during IPGTTs.
a, Left, inhibition of mouse IDE activity by low potency diastereomers of 6bK (right). The stereocentres altered in each compound relative to those of 6bK are labelled with asterisks. b–e, In dose optimization experiments, the effects of 6bK (40 to 90 mg kg−1, n = 5 and 4, respectively) were compared with equal doses of either weakly active stereoisomer epi-C-6bK (n = 5, panel b) or inactive stereoisomer bisepi-6bK (n = 5, panel c) and vehicle controls (n = 5) in IPGTTs using lean and obese mice. d, DIO mice treated with low doses of 6bK (40 mg kg−1, n = 7) responded to IPGTT in either of two ways: improved glucose tolerance throughout the experiment (n = 3) or a hyperglycaemic ‘rebound’ as described in the main text (n = 4), suggesting this dose is too low to achieve a consistent ‘rebound’ phenotype (note the large error bars). e, DIO mice treated with high doses of 6bK (3.5 mg per animal, 90 mg kg−1, n = 5) respond similarly to Fig. 2e (2 mg per animal, 60 mg kg−1), but the weak activity observed for bisepi-6bK (IC50 > 200 µM) using a matching dose (90 mg kg−1, n = 5) compared to vehicle alone (n = 5) suggests that 60 mg kg−1 (2 mg per animal) is the appropriate dose for DIO mice experiments. All data points and error bars represent mean ± s.e.m. Statistics were performed using a two-tail Student’s t-test, and significance levels shown in the figures are *P < 0.05 versus vehicle control group; **P < 0.01 versus vehicle control group. Data shown in b–e are representative of two or more independent studies.