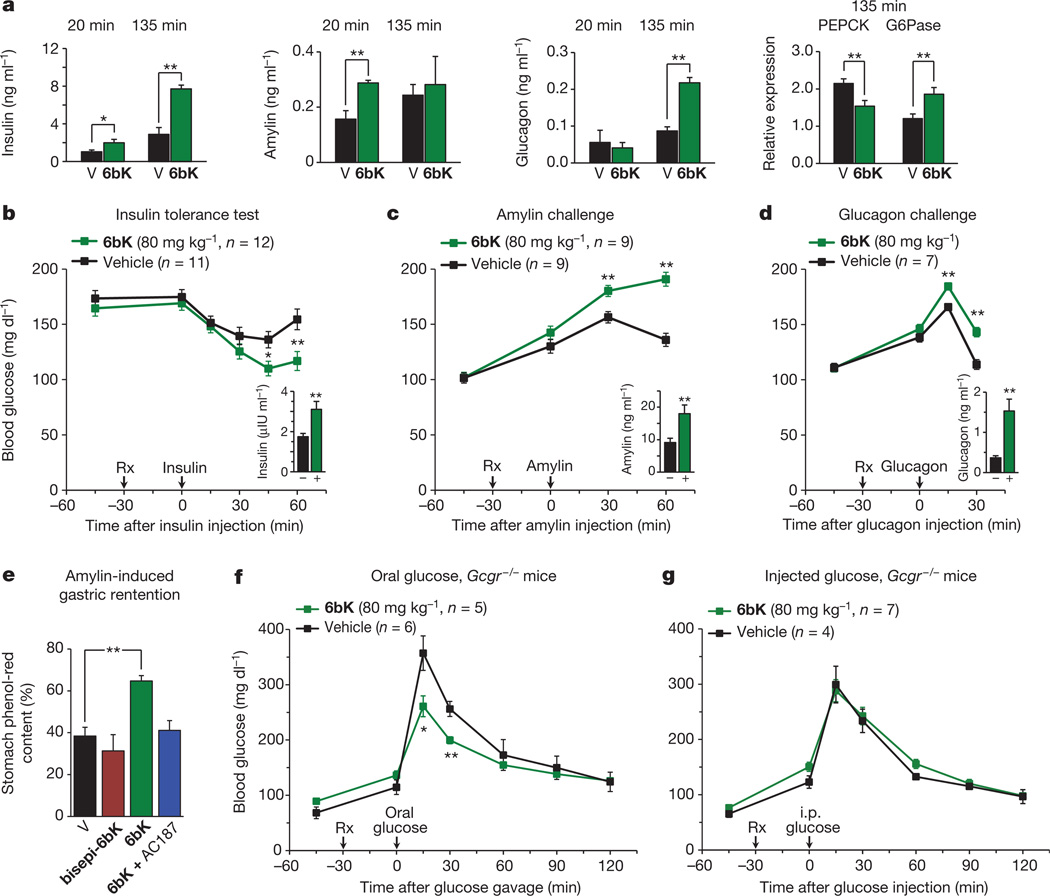
Figure 3. Acute IDE inhibition affects the abundance of multiple hormone substrates and their corresponding effects on blood glucose levels.
a, Plasma hormone measurements at 20 min and 135 min after i.p.-injected glucose tolerance tests (IPGTTs) on DIO mice (Fig. 2e) and RT–PCR analysis of DIO liver samples collected at 135 min after IPGTT. RT–PCR reveals 50% higher glucose-6-phosphatease (G6Pase) and 30% lower phosphoenolpyruvate carboxykinase (PEPCK) transcript levels for the 6bK-treated cohort (6bK; n = 7) versus vehicle-only controls (V; n = 7). b–d, Blood glucose responses and injected hormone abundances in lean mice 30 min after treatment with 6bK or vehicle alone. b, Insulin (0.25 U kg−1, subcutaneous) after 5-h fast. c, Amylin (250 µg kg−1, subcutaneous) after overnight fast. d, Glucagon (100 µg kg−1, subcutaneous) after overnight fast. Trunk blood was collected at the last time-points for plasma hormone measurements (insets; IU, international units). e, Acute IDE inhibition slows gastric emptying through amylin signalling. Fasted WT mice were given oral glucose supplemented with 0.1 mg ml−1 phenol red 30 min after treatment with 6bK alone (n = 6), 6bK co-administered with the amylin receptor antagonist25 AC187 (3 mg kg−1 i.p., n = 6), vehicle alone (V; n = 6) or inactive bisepi-6bK (n = 4). f, g, G-protein-coupled glucagon receptor knockout mice (Gcgr−/−) treated with IDE inhibitor 6bK display altered glucose tolerance relative to vehicle-treated mice if challenged with oral glucose (3.0 g kg−1; f) but not i.p. injected glucose (1.5 g kg−1; g). Data: mean ± s.e.m.; *P < 0.05, **P < 0.01 in two-tail Student’s t-test. Data shown in a–e are representative of two or more independent studies. Studies using Gcgr−/− knockout mice were performed once.