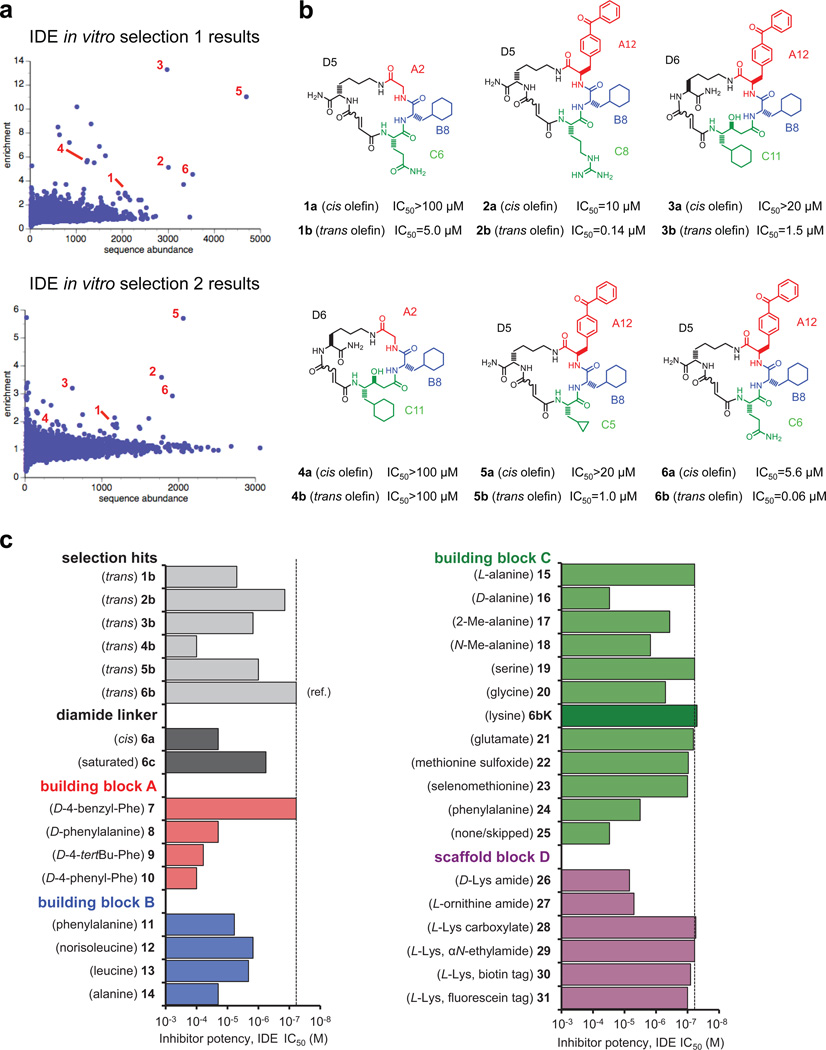
Extended Data Figure 1.
a, Enrichment plots from two independent in vitro selections against N-His6-mIDE using a 13,824-membered DNA-templated macrocycle library7,8. The numbers highlight compounds enriched at least twofold in both selections. b, Structures of IDE-binding macrocycles 1–6 decoded from DNA library barcodes corresponding to building blocks A, B, C and D (Fig. 1). The cis and trans isomers are indicated by suffices a and b respectively (for example, 1a and 1b). The two isomers were synthesized as previously reported18,19 and separated by HPLC. c, IDE inhibition potency of trans hits 1b to 6b compared to 30 structurally related 6b analogues in which the linker, scaffold and the three building blocks were systematically varied. IDE inhibition activity was assayed by following cleavage of the fluorogenic peptide substrate Mca-RPPGFSAFK(Dnp)-OH.