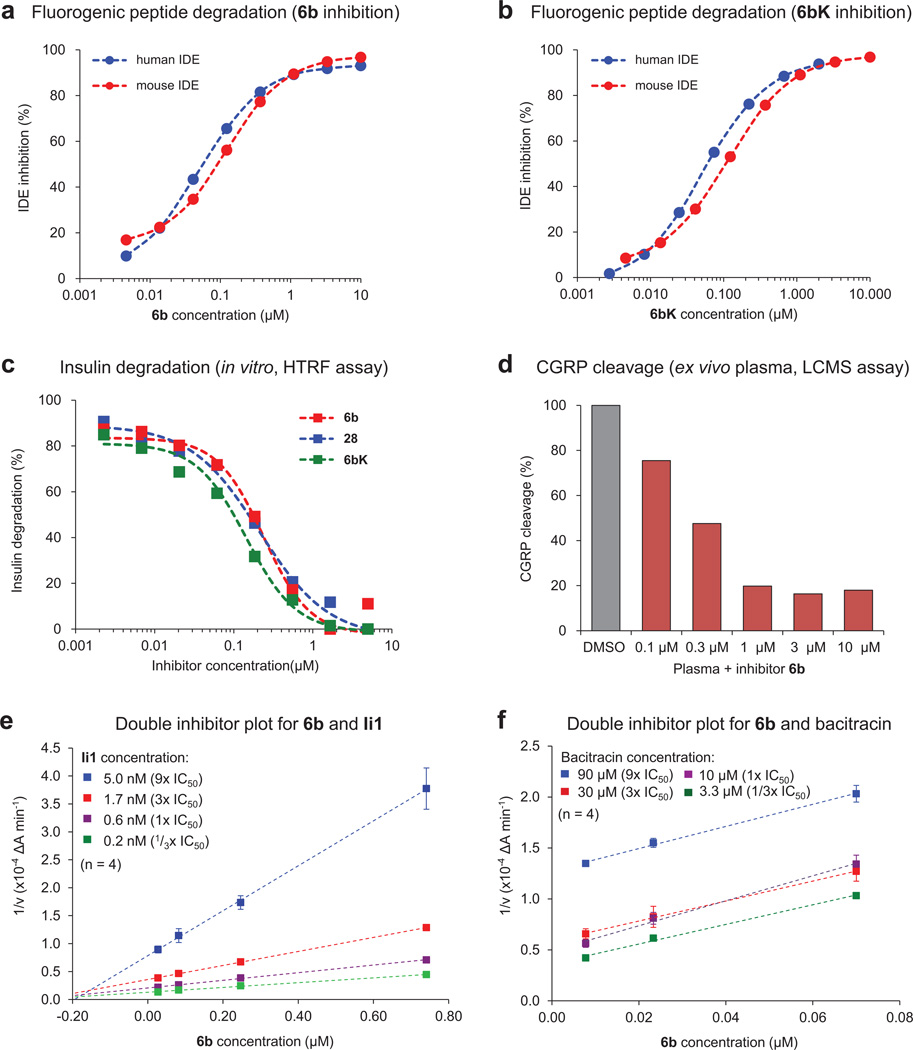
Extended Data Figure 2. Inhibition of human and mouse IDE activity in multiple assays.
a, b, Human IDE shares 95% sequence homology with mouse IDE13, and cleavage of the fluorogenic substrate peptide Mca-RPPGFSAFK(Dnp)-OH by human and mouse IDE is inhibited with similar potency by 6b (a) and 6bK (b). c, Homogeneous time-resolved fluorescence (HTRF) assay measuring degradation of insulin by IDE in the presence of 6b, 6bK and analogue 28 (Extended Data Fig. 1). d, LC-MS assay for ex vivo degradation of CGRP (10 µM) by endogenous IDE in mouse plasma in the presence of 6b. e, f, Biochemical assays suggesting that 6b binds a site in IDE distinct from the conventional peptide substrate binding site known to bind substrate mimetic Ii1. Yonetani–Theorell double inhibitor plots of IDE activity in the presence of 6b and Ii1 (panel e), or 6b and bacitracin (panel f). Crossing lines indicate synergistic and independent binding of inhibitors, while parallel lines indicate competition for binding to the enzyme.