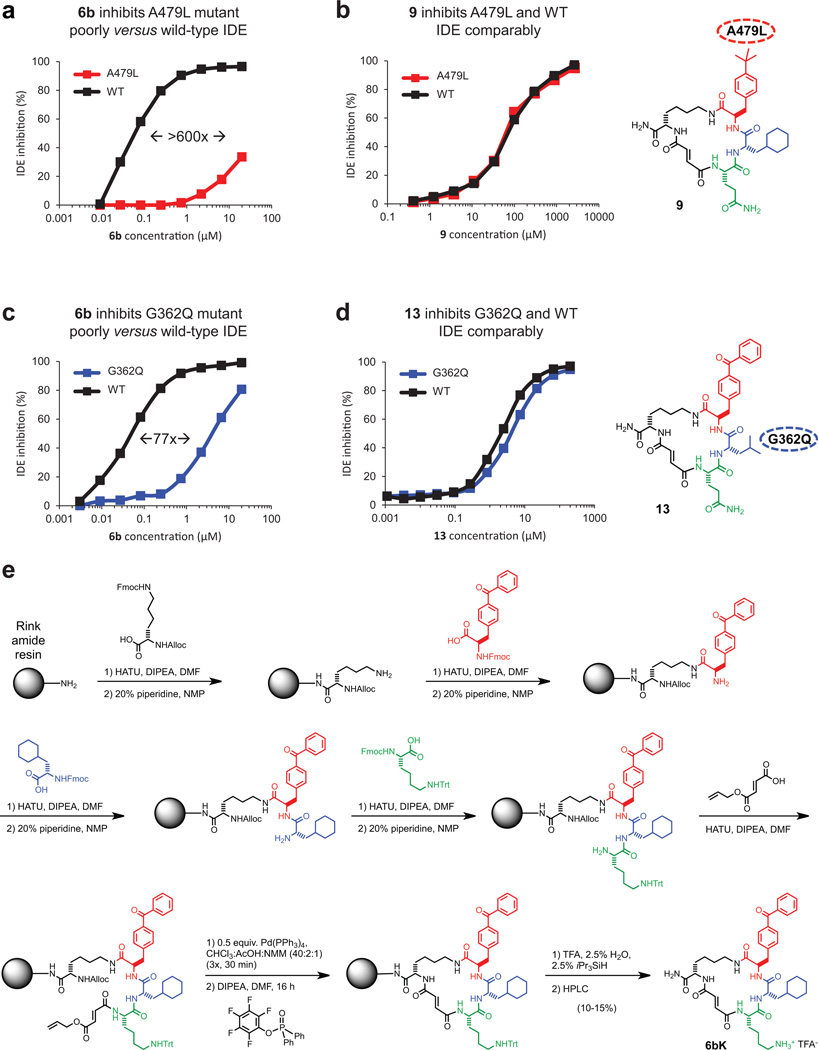
Extended Data Figure 4. Small-molecule/enzyme mutant complementation study to confirm the macrocycle binding site and placement of the benzophenone and cyclohexyl building-block groups.
a, IDE mutant A479L is inhibited by 6b > 600-fold less potently compared to wild-type IDE. b, Analogue 9, in which the p-benzoyl ring is substituted for a smaller t-butyl group, inhibits A479L IDE and WT IDE comparably. c, Similarly, IDE mutant G362Q is inhibited 77-fold less potently by 6b compared with WTIDE. d, Analogue 13, in which the L-cyclohexyl alanine side chain was substituted with a smaller L-leucine side chain, inhibits G362Q IDE and WT IDE comparably. The full list of IDE mutants investigated is shown in Supplementary Table 5. e, Synthetic scheme for 6bK, also used for synthesis of 6bK analogues.