Figure 5.
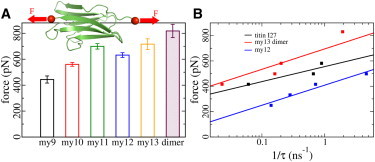
Structural robustness of Ig domains. (A) Forces and standard errors for the unfolding of all five myomesin Ig domains and the detachment force of the myomesin dimer. These forces were obtained by subjecting individual Ig domains (or the my13 dimer) to constant loading applied to the termini (red spheres) in opposite directions (red arrows). (B) Stability comparison of my12, the my13 dimer, and the titin I27 domain. The my13 dimer mechanically outperforms other Ig domains. Rupture times τ were obtained from simulations at varying constant forces. (Solid lines) Bell’s model (40) fits to the data, with F ∼ 1/Δx≠ × ln 1/τ. The dissociation of the my13 dimer shows a similar distance to the transition state along the pulling direction (Δx≠ = 0.36 nm) as my12 unfolding (Δx≠ = 0.51 nm).
