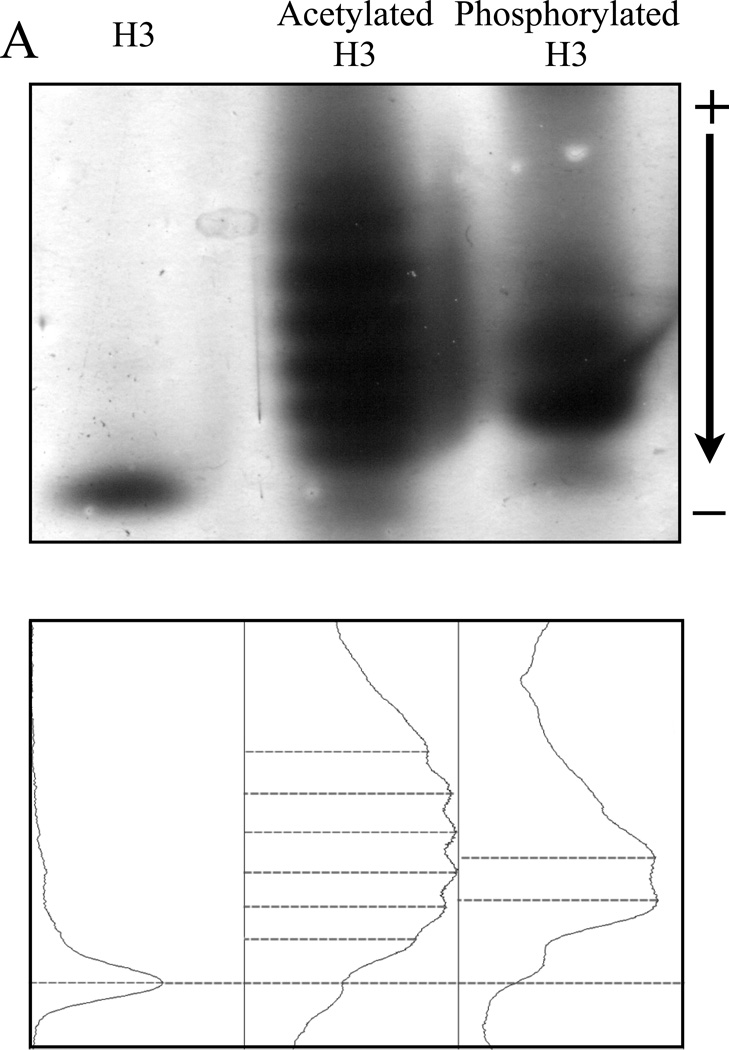
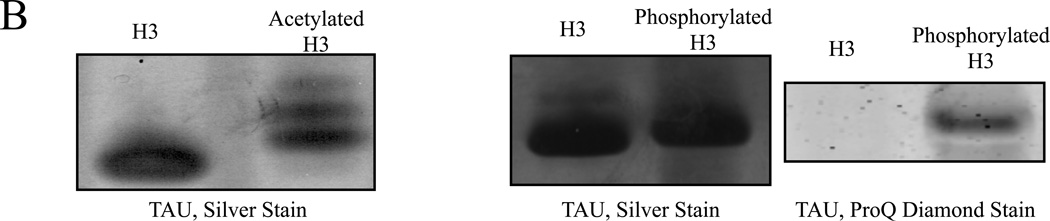
Figure 3. Both acetylated and phosphorylated histone H3 isoforms can be resolved by NUT-PAGE, whereas TAU-PAGE can only resolve the acetylated H3 species.
(A) 15% NUT-PAGE was used to separate the positively charged histone H3. The densitometric traces (bottom) of the silver-stained histone H3 species were generated by ImageJ (http://rsbweb.nih.gov/ij/). Both acetylation and phosphorylation reduce the net positive charge on histone H3, resulting in multiple bands that display reduced mobility in the gel matrix. Note that histone H3 is a basic protein, which thus was run from annode to cathode. (B) Triton-acetic acid-urea (TAU) gel does not resolve phosphorylated histone H3. A conventional TAU gel (15%) was used to resolve different H3 isoforms. The left panel shows silver stained unmodified and acetylated histone H3 after electrophoresis. Acetylated species of histone H3 migrate at a slower rate, with comparable resolution to NUT-PAGE. The right panel shows unmodified and phosphorylated histone H3 stained by either silver stain or the phospho-specific ProQ Diamond stain following TAU-PAGE. Phosphorylated histone H3 displays only a marginal decrease in mobility. ProQ Diamond staining confirmed phosphorylation was present on the modified protein.