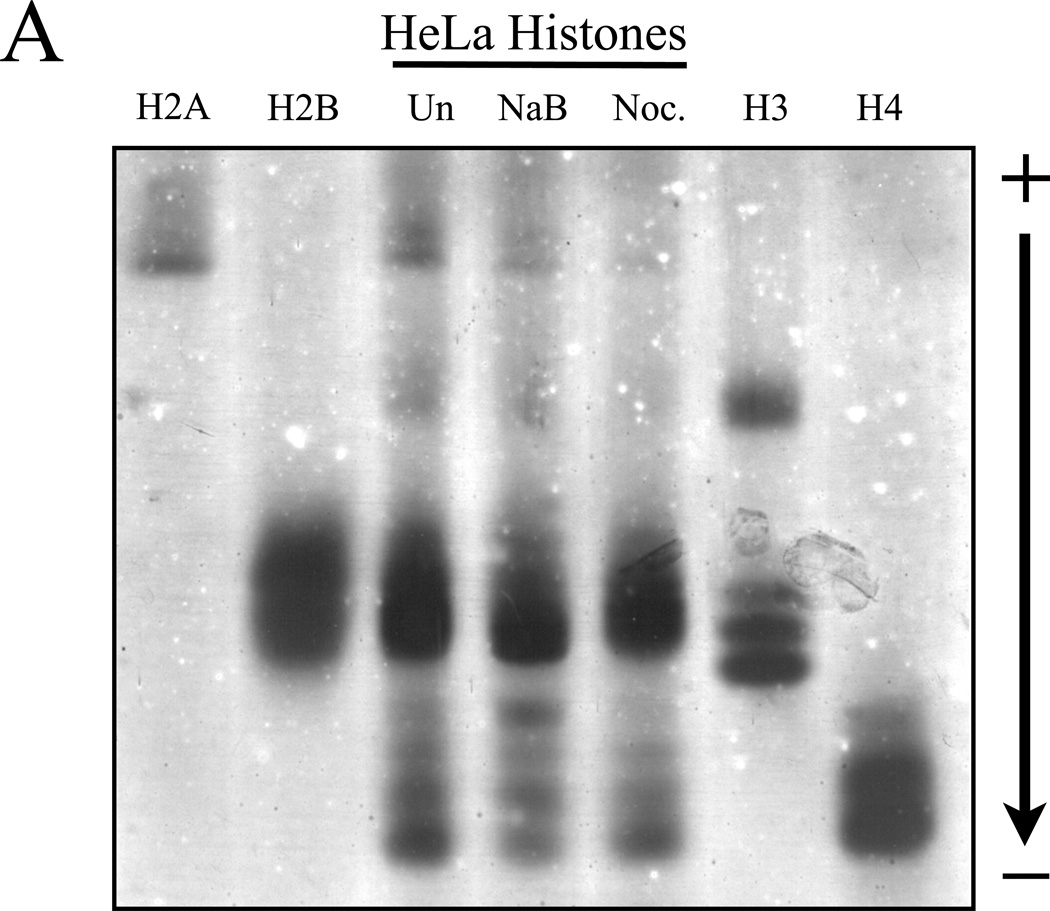
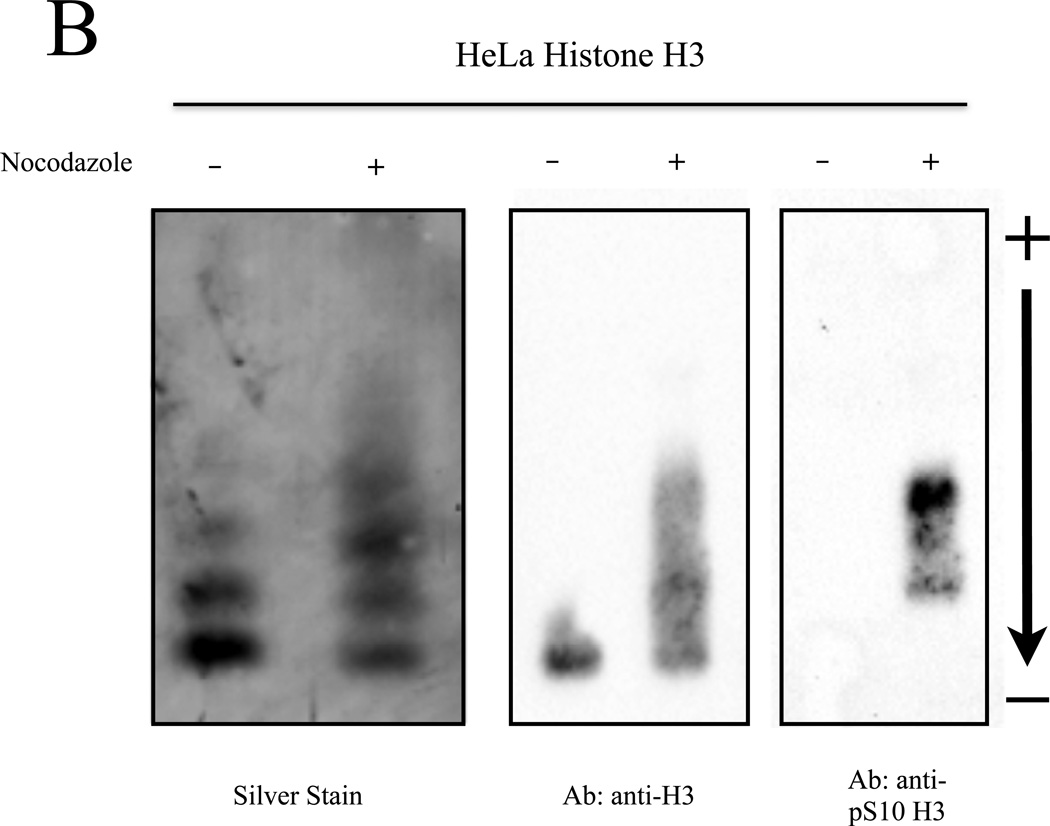
Figure 4. Post-translationally modified histones isolated from cell culture can be resolved by NUT-PAGE.
(A) HeLa cells were treated with sodium butyrate to induce hyperacetylation or nocodazole to block progression through mitosis and enrich for phosphorylated serine 10 of histone H3. Histone proteins were then isolated and separated by NUT-PAGE. Sodium butyrate treatment resulted in increased histone H4 acetylation, as can be seen by a decreased mobility of multiple bands resulting from the loss of charge. Histone H3 phosphorylation cannot be noted due to co-migration of histones H2B and H3. Purified chicken histones were used as mobility references for each core histone protein. (B) Histone H3 was isolated from crude histones by RP-HPLC and modified species of histone H3 were resolved by NUT-PAGE and visualized by silver staining. Histone H3 collected from untreated HeLa cells migrates as three distinct bands, while histone H3 from nocodazole treated HeLa cells displayed additional bands with overall lowered mobility in NUT-PAGE, consistent with the fact that metaphase H3 is phosphorylated at Ser10. Western analysis using an antibody against H3 phosphorylated at Ser10 confirmed that phosphorylation is associated with retarded gel mobility of NUT-PAGE.