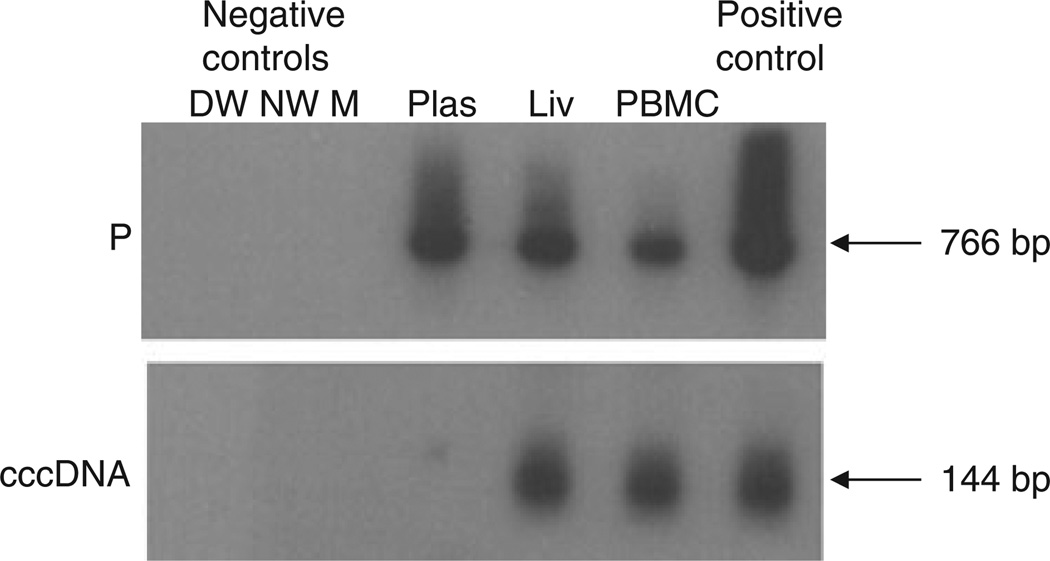
Fig. 3.
Detection of HBV P gene and HBV covalently closed circular DNA (cccDNA) amplicons in different compartments of HBV occurrence in a patient (Case 6) treated with emtricitabine (FTC) and tenofovir (TDF). The detection of HBV DNA in plasma (Plas), liver (Liv) and peripheral blood mononuclear cells (PBMC) by PCR/nucleic acid hybridization is illustrated, although HBV DNA was undetectable in serum by standard clinical assays. All reactions were run in parallel with strict contamination controls, as described in legend to Fig. 1. The size of the expected direct HBV P gene amplicons (766 bp) and the HBV cccDNA amplicons (144 bp) is indicated to the left of each panel.