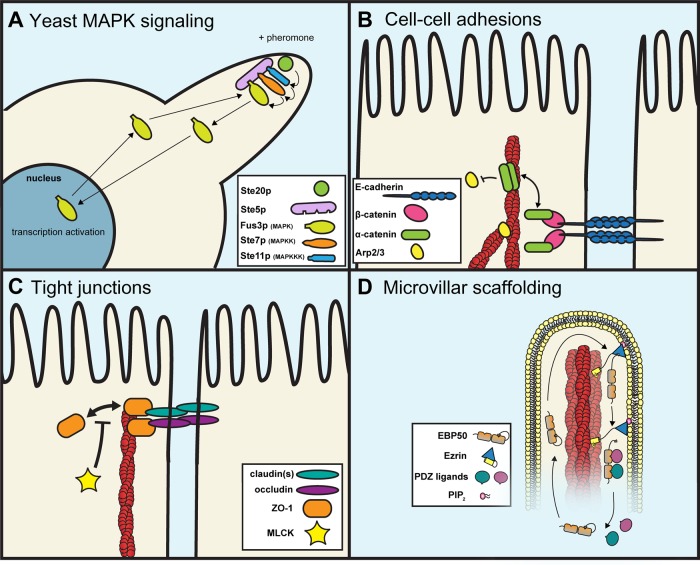
FIGURE 1:
Examples of dynamic scaffolding protein complexes once thought to be stable. (A) Ste5p, a scaffold for the MAPK cascade during mating in budding yeast, brings together Ste11p, Ste7p, and Fus3p at mating projection tips. Fus3p can rapidly shuttle between the cytoplasm and nucleus to activate transcription and also provides negative feedback via phospho-mediated inhibition of Ste5p activity. (B) The linking protein α-catenin is a critical component of cell–cell adhesions. As a monomer, it binds to the E-cadherin–β-catenin complex. As a homo-dimer, α-catenin can bind to F-actin (red) and blocks Arp2/3 binding, thereby preventing local actin polymerization. (C) The MAGUK family member ZO-1 plays an essential role in tight junction barrier function. It binds to the C-terminal tails of claudins and occludins and links them to the underlying F-actin. The linkage provided by ZO-1 is transient, as it freely exchanges with the cytoplasm. This dynamic exchange is suppressed by MLCK activity. (D) The PDZ scaffolding protein EBP50 provides a critical linkage between PDZ ligands and ezrin in microvilli on the apical surface of epithelial cells. The EBP50 tail has a high-affinity association with ezrin and is stable when not bound to PDZ ligands. On PDZ ligand binding, the EBP50 tail–ezrin interaction becomes dynamic, and EBP50 rapidly exchanges with the cytoplasm.