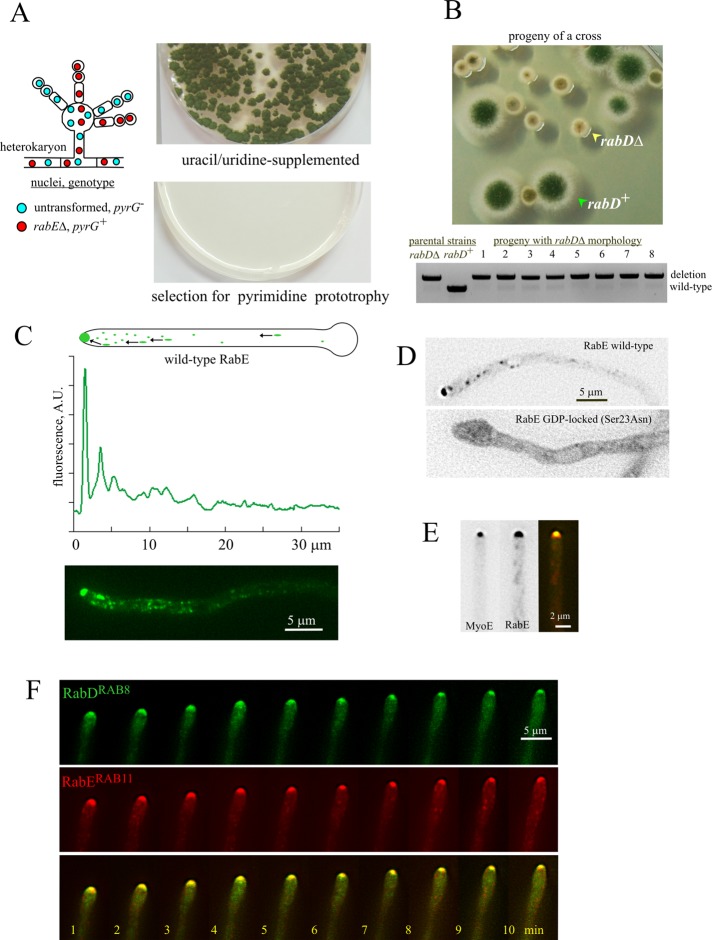
FIGURE 1:
The exocytic A. nidulans RABs, RabERAB11 and RabDRAB8. (A) Heterokaryon rescue test (Osmani et al., 2006) demonstrating that rabE is essential. (B) Progeny of a cross between rabD+ and rabD∆ strains showing the rabD∆ growth phenotype, and diagnostic PCR establishing that eight random progeny with this growth phenotype contained the deletion allele. (C) Distribution of GFP-RabE. Expression was driven by the rabEp (strain MAD4120; Supplemental Table S1). The top linescan shows its marked polarization. (D) GDP-locking Sec23Asn substitution delocalizes RabERAB11 to a cytosolic haze and to a faintly labeled network of endomembranes. Wild-type and mutant GFP-RabERAB11 (strains MAD4072 and MAD4271) were expressed under the control of the alcAp, which was induced for 3 h after shifting cells to medium containing ethanol. Despite the fact that this regime results in GFP-RabERAB11 overexpression (see text and Supplemental Figure S2), the subcellular distribution of the wild-type fusion protein is indistinguishable from that attained with the natural rabE promoter. Images are middle planes of Z-stacks that have been contrasted with the “unsharp mask” filter of MetaMorph to maximize detection of endomembranes in the mutant. (E) MyoE-GFP colocalizes with mCherry-RabE at the SPK. Images of strain MAD4403, in which MyoE was endogenously tagged with GFP and mCherry-RabERAB11 expression was driven by the alcAp promoter. Cells were cultured on fructose to obtain similar levels of fluorescence, which permitted simultaneous GFP and mCherry channel recording using a Dual View. Note that the localization patterns of mCherry-RabERAB11 and GFP-RabERAB11 are indistinguishable (see Materials and Methods and Supplemental Figure S2). (F) GFP-RabD and mCherry-RabE colocalize at the SPK in a growing hypha. This time series was obtained with strain MAD4156, in which both GFP-RabDRAB8 and mCherry-RabERAB11 were expressed from single-copy alcAp-driven constructs targeted to the pyroA and argB loci, respectively.