Table 1.
Highly diastereo- and enantioselective one-pot aldehyde allylation and crotylation reactions with diaminophenol 6
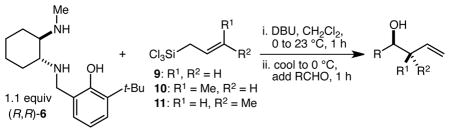
| ||||||
|---|---|---|---|---|---|---|
| entry | silane | RCHO | product | yield (%)a | dr | ee (%) |
| 1 | 9 |
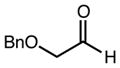
|
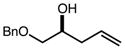
|
83 | - | > 99 |
| 2 | 9 |
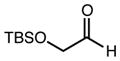
|
|
81b | - | 98 |
| 3 | 10c |
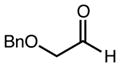
|
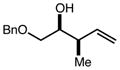
|
94b | 98:2d | 96 |
| 4 | 11e |
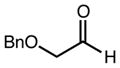
|
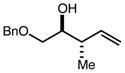
|
88 | 94:6d | 97 |
| 5 | 10c |
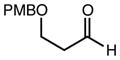
|
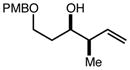
|
92 | > 98:2d | 93 |
| 6 | 9 |
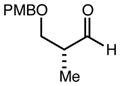
|
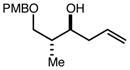
|
82 | 98:2f | - |
| 7 | 10c |
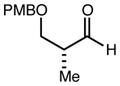
|
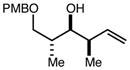
|
82 | 92:8g | - |
| 8 | 11e |
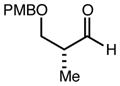
|
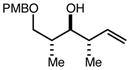
|
80 | 93:7g | - |
| 9 | 9 |
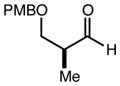
|
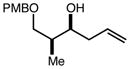
|
80 | 98:2f | - |
| 10 | 10c |
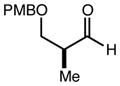
|
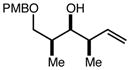
|
80 | 94:6g | - |
| 11 | 11e |
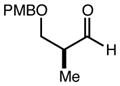
|
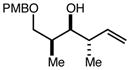
|
80 | 95:5g | - |
| 12 | 9 |
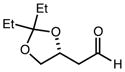
|
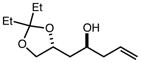
|
87b | 96:4f | - |
Isolated yield of purified products.
A modified workup and isolation procedure was employed.
The geometric purity of the cis-crotyltrichlorosilane employed was measured to be >99:1 by 1H NMR spectroscopic analysis.
Syn:anti diastereoselectivity as measured by 1H NMR spectroscopic analysis.
The geometric purity of the trans-crotyltrichlorosilane employed was measured to be 95:5 by 1H NMR spectroscopic analysis.
Aldehyde diastereoface selectivity as measured by 1H NMR spectroscopic analysis.
Ratio of the major diastereomer to the sum of the minor diastereomers as measured by HPLC and 1H NMR spectroscopic analysis. See the supporting information for details.
