Abstract
Introduction
With the advent and availability of targeted therapy, the treatment of advanced/metastatic renal cell carcinoma (RCC) underwent a drastic change in 2005. The impact of this change on clinical outcome, within the population has not been studied. The aim of this study was to evaluate the overall survival (OS), prior to, and post availability of targeted therapy, for advanced RCC cases in the population-based Surveillance, Epidemiology and End Results (SEER) cancer registry.
Methods
All advanced (regional and distant stage) RCC cases diagnosed within the 2000–2008 time periods were included. Since SEER does not report the exact therapy, and as targeted therapy was initially approved in 2005, we evaluated and compared the OS outcomes of advanced RCC cases diagnosed between the years, 2000–2003 (pre targeted therapy era) with that of those diagnosed between 2005–2008 (targeted therapy era).
Results
There was a significant improvement in OS for advanced RCC patients treated in the targeted therapy era (N= 12,330) compared to those treated in the pre-targeted therapy era (N=11,565) (Median OS 20 months vs. 15 months, p= 0.0006). Multivariate analysis revealed that the pre-targeted therapy time period, age over 65 years, black race, and lack of nephrectomy were predictors of a shorter OS.
Conclusion
In univariate and multivariate analysis, targeted therapy demonstrated improvement in OS. Increasing access to targeted therapies is likely to improve outcomes in advanced RCC.
Keywords: kidney cancer, epidemiology, SEER database, racial disparity, nephrectomy
INTRODUCTION
In the year 2013, an estimated 65,150 new kidney and renal pelvis cancers will be diagnosed in the United States. andabout 13,680 will die of the disease[1]. The predominant cause of mortality in kidney cancer is advanced or metastatic disease. Withinthe 2001–2007 time period, approximately 30 – 36% of kidney and renal pelvis cancers presented with advanced stage disease (regional ormetastatic disease) at initial presentation [1]. From 1990 to 2006 there has been a reported 6.8% absolute reduction in RCC mortality [2]. Early detection of RCC in the localized stage, and increased rates of nephrectomy, are likely reasons for the decreased disease related mortality in the localized stage. In advanced disease the most likely cause is the availability of better systemic therapies.
Prior to targeted therapy, immunotherapy [3,4] was the only systemic therapy indicated for advanced kidney cancer over the previous two decades. Targeted therapy based on the principle of vascular endothelial growth factor (VEGF) inhibition was approved by the Food and Drug Administration, for routine use in 2005 [5–7]. Subsequently mammalian target of rapamycin (mTOR) pathway inhibition therapies were also approved [8,9]. These therapies demonstrated statistically significant progression free survival benefit in randomized trials, and temsirolimus also demonstrated overall survival benefit. The availability of these agents changed the therapeutic dynamics in RCC, inducing a paradigm shift, from lack of effective therapy; to that of tolerable and active therapy which is applicable to the majority of RCC patients. However outside of clinical trial data, the impact of these expensive and somewhat toxic therapies in a population based sample of advanced RCC is unknown. The Surveillance, Epidemiology and End Results 17 (SEER 17) cancer registry was thought to be the ideal database to explore this impact, since it is likely to reflect on the general population based management of advanced RCC.
METHODS
SEER database
The National Cancer Institute’s SEER program is a premier resource for cancer statistics in the United States. It collects information on cancer incidence and mortality from specific geographic and demographic areas representing 28% of the US population. The data collected include patient demographics, type of cancer, tumor characteristics, the extent of disease at time of diagnosis, and type of treatment received for the first course of therapy. Follow-up on each patient is conducted annually to assess current vital status.
All individuals diagnosed with regionally advanced (lymph node involvement) or metastatic clear cell and non-clear cell RCC from January 1, 2000 to December 31, 2008 were included. Unknown race and Autopsy/DCO (death certificate only) cases were excluded. Histologically confirmed RCC cases with regional/metastatic stage were selected, and data regarding patient demographics (sex, race, age), tumor histology, nephrectomy status, disease stage (regional/metastatic), initial therapy and overall survival were collected. We restricted our study to the advanced RCC (regional and distant per SEER staging categories) cases only, and excluded localized disease, as systemic therapy is currently approved only in the locally advanced, unresectable or metastatic stages of RCC.
Study objectives
The primary objective of the study was to evaluate and compare the OS of regional and distant RCC cases diagnosed in the targeted therapy era to that of the pre-targeted therapy. The exact type of therapy administered to the patient is not captured by the SEER registry. However, as targeted therapies became available only since the later part of 2005, we considered 2000–2003 as the pre-targeted therapy era and 2005–2008 as the targeted therapy era. We excluded the patients from the year 2004 to avoid overlap with any clinical trial population that may have received targeted therapy. The 2000–2003 and 2005–2008 time periods are hereinafter addressed as the “pre-targeted” and “targeted therapy” eras, respectively. We compared OS by the above time periods and stratified by gender (male, female), race (black, non-black), age (<65, >/=65), stage (regional, distant) and nephrectomy status (yes, no).
The other objective was to evaluate the hazard ratio for risk of death in the targeted and pre targeted time periods, when adjusted for the important demographic, disease related and treatment related factors available in the SEER database such as, age, race, histology, stage and nephrectomy.
Statistical analysis
All statistical tests were performed using SAS 9.2 software. Chi-square test was used to determine relationship between patient characteristics and time period. To compare the difference in proportions of the patients’ characteristics between the two time periods. The Z-test and Wilcoxon-Mann-Whitney test, a non-parametric test were used to compare the difference in the median ages between the two time periods.
The Kaplan-Meier survival estimates method was used for generating the survival curves, and for computing the log-rank test and the survival proportions. Multivariate Cox-Proportional hazards models were used to assess the effects of prognostic factors, and estimate hazard ratios of the different variables on OS, 95% confidence intervals (CI) and p-values.
RESULTS
Patient Characteristics
In the SEER 17 data, 11,565 patients were diagnosed in the pre-targeted therapy era, and 12,330 patients in the targeted therapy era. No major differences in distribution of patient characteristics were identified between the two groups (Table 1). The median age was 66 years and the majority of the patients were white with black patients constituting approximately 8% of the population. About two-thirds of the patients were male and the predominant histology was clear cell type (80%). Similar proportions of patients (about 60%) underwent nephrectomy in each of the time periods.
Table 1.
Baseline patient characteristics in the SEER 17 registries
| Variable | 2000–2003 | 2005–2008 |
|---|---|---|
| N (%) 11565 |
N (%) 12330 |
|
| Median age (yrs) | 66 | 66 |
| Age Group | ||
| <65 | 5328 (46.1) | 5803 (47.1) |
| >/=65 | 6237 (53.9) | 6527 (52.9) |
| Race | ||
| White | 9940 (85.9) | 10461 (84.8) |
| Black | 1035 (8.9) | 1017 (8.3) |
| Other | 569 (4.9) | 814 (6.6) |
| Unknown | 21 (0.2) | 38 (0.3) |
| Stage | ||
| Regional | 5404 (46.7) | 5902 (47.9) |
| Distant | 6161 (53.3) | 6428 (52.1) |
| Gender | ||
| Female | 3961 (34.3) | 4055 (32.9) |
| Male | 7604 (65.8) | 8275 (67.1) |
| Histology | ||
| Clear cell | 9296 (80.4) | 9787 (79.4) |
| Non-clear cell | 2269 (19.6) | 2543 (20.6) |
| Nephrectomy | ||
| Yes | 7063 (61.1) | 7667 (62.2) |
| No | 4502 (38.9) | 4663 (37.8) |
Overall Survival Data
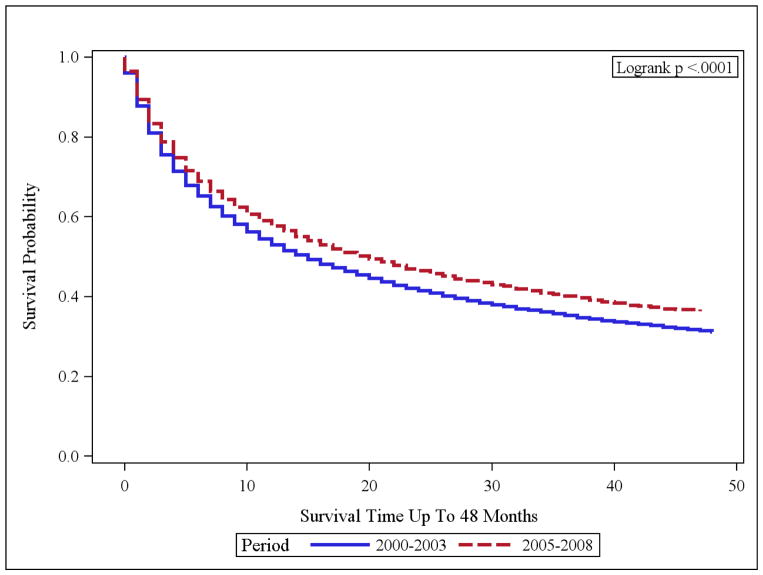
In the targeted therapy era, advanced RCC cases (regional and distant disease) had a statistically significant improvement in OS than the pre-targeted therapy group (p <0.001) [Figure 1]. The median OS were 15 and 20 months in the pre-targeted and the targeted therapy eras, respectively. When distant cases were analyzed separately, only a 2 month improvement in median survival from 5 to 7 months was noted (p<0.001) [Figure 2, Table 2]. The regional cases in the targeted therapy era also demonstrated an OS improvement as compared to the pre-targeted therapy time period, however median survivals for both these subpopulations have not been reached. The 3 year survival rates in the cases with regional disease only demonstrated a 5.5% improvement from 67.9% in the 2000–2003 time period, to 73.4% in the 2005–2008 time period.
Figure 1.
OS of Advanced RCC [Regional and Distant] Time periods: 2000–2003 vs. 2005–2008
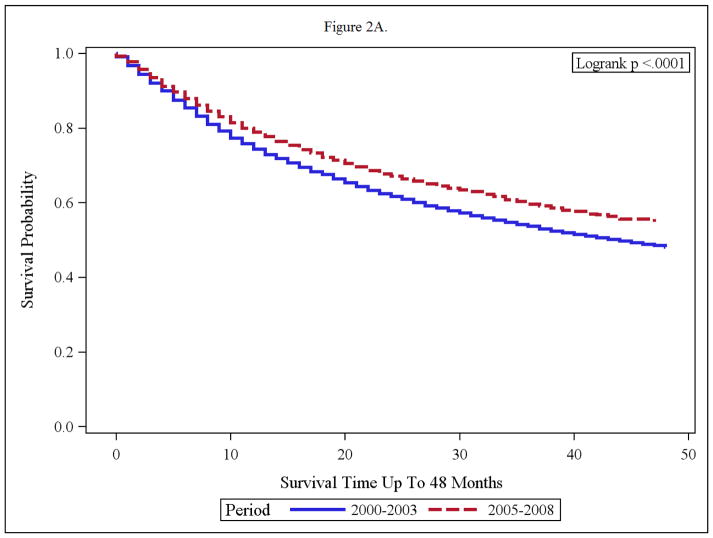
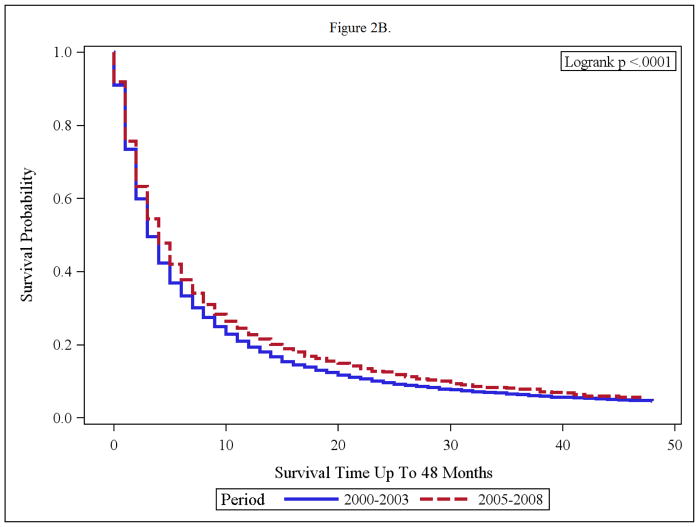
Figure 2.
(A and B): Overall Survival of Adult RCC (Regional/Distant) with nephrectomy (A) and without nephrectomy (B) in the time periods; 2000–2003 and 2005–2008.
Table 2.
Survival data in advanced RCC cases, SEER 17 2000–2008
| 1 year OS | 2000–2003 | 2005–2008 |
|---|---|---|
| All | 52.9% (52%–54%) | 57.5 (57% – 59%) |
| Regional (Local LN involvement) | 80.2% (79% – 81%) | 83.6% (83% – 85%) |
| Distant | 29.1% (28% – 30%) | 34.1% (33% – 35%) |
| 3 year OS | P <0.001 | |
| Distant disease | 13.3 % (12.4–14.2) | 16.2 % (14.9–17.6) |
| Regional Disease | 67.9 % (66.4–69.3) | 73.4 % (71.5–75.1) |
Impact of Nephrectomy on Overall Survival
The impact of nephrectomy was explored in the two timeframes. Advanced RCC cases without nephrectomy, had minimal benefit from targeted therapy with median survival of 4 months and 3 months in the targeted and pre-targeted eras respectively. (Fig 2). The median OS in nephrectomy patients, in the pre-targeted therapy era, was 44 months, and has not been reached yet in the targeted therapy era (Figure 2 A and B).
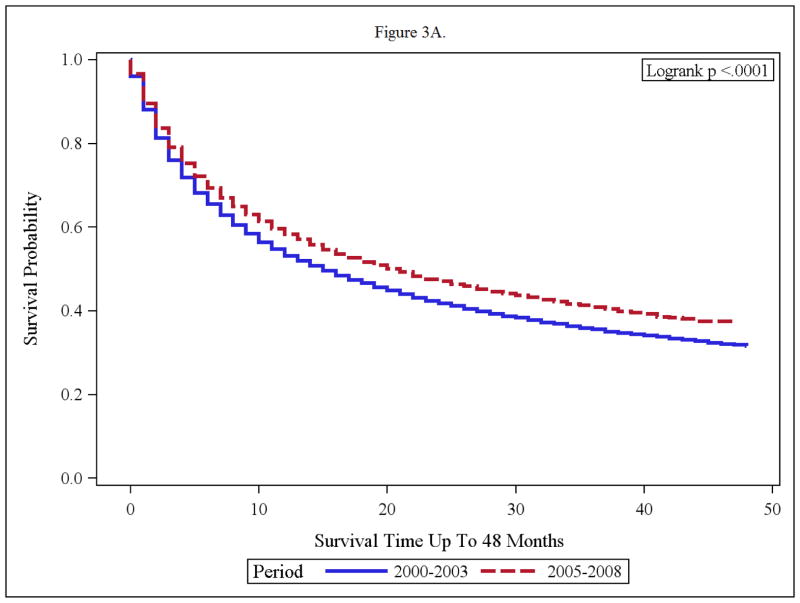
Impact of Race on Overall Survival
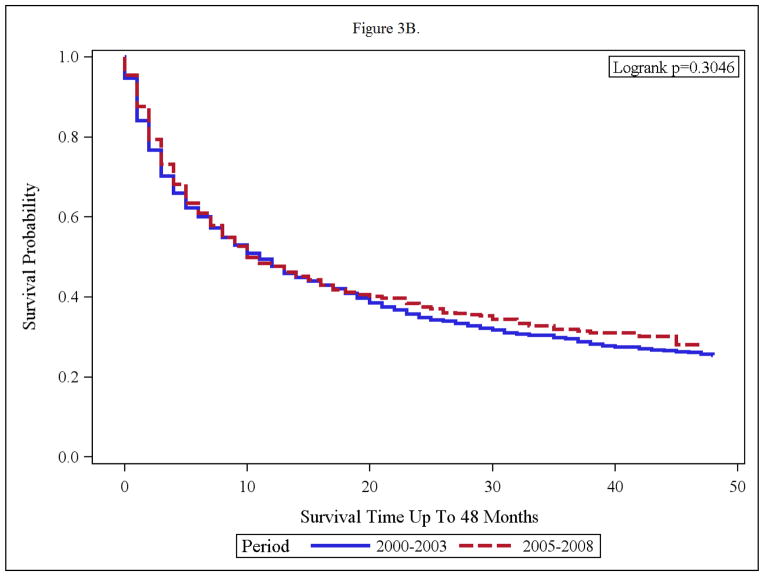
Compared to the pre-targeted therapy era, white patients in the targeted therapy era, had a clinically and statistically significant improvement in OS (p< 0.0001, median OS of 15 months to that of 21 months). The black patients appeared to derive no OS benefit in the targeted therapy era. In fact, median OS was 10 months in the targeted time period and median OS was 11 months in the pre targeted therapy era (p=0.3046). [Figures 3 A and B].
Figure 3.
(A and B): Overall Survival of Advanced RCC Patients Within Treatment Time periods; 2000–2003 and 2005–2008 for Whites (A) and Blacks (B)
Histology and Survival
Patients with clear cell (CC) histology demonstrated a longer survival as compared to those with non-clear cell histology (non-CC). The extent of OS improvement was comparable in both the time periods [Table 3]. The median OS in the subgroups with CC in targeted and pre targeted therapy eras were 22 and 17 months respectively and in those with non CC histology were 13 and 9 months respectively [Table 3].
Table 3.
Prognostic variables and Adjusted Hazard Ratios Multivariable analysis:
| Variable | 2000–2003 HR (95% CI) P value | Med. Survival (Months) | 2005–2008 HR (95% CI) P value | Med. Survival (Months) |
|---|---|---|---|---|
| Race (Black vs White) | 1.11 (1.03–1.19) 0.006 | 11 Vs 15 | 1.09 (1.01–1.19) 0.04 | 10 Vs 21 |
| Age group (>/=65 vs <65) | 1.25 (1.19–1.30) <0.001 | 11 vs 21 | 1.27 (1.21–1.34) <0.001 | 14 vs 28 |
| Nephrectomy (No vs Yes) | 2.74 (2.59–2.89) <0.001 | 3 Vs 44 | 3.29 (3.08–3.51) <0.001 | 4 Vs NR |
| Stage (Distant vs Regional) | 2.82 (2.66–2.99) <0.001 | 5 Vs NR | 2.97 (2.76–3.19) <0.001 | 7 Vs NR |
| Histological type (NCC vs CC) | 1.32 (1.25–1.39) <0.001 | 9 Vs 17 | 1.32 (1.24–1.41) <0.001 | 13 Vs 22 |
Multivariate Analysis of Prognostic Variables and Survival
In a multivariate analysis of known prognostic factors, the variable, “targeted therapy era” persisted in demonstrating improved outcome. A hazard ratio (HR) of risk of death of 1.16 (95% CI 1.116–1.195, p= 0.0001) was noted for the risk of death of patients treated between the time periods 2000–2003 in comparison with those treated between 2005–2008. The other variables analyzed included, age, race, stage, nephrectomy status and histology. All of these variables had a significant prognostic effect on survival in both the pre-targeted and targeted therapy eras. The prognostic variables and the adjusted HR are depicted in Table 3. Risks of death by race, age group (65+ vs. <65) and histology, did not vary between the two time periods. However, in the patients without nephrectomy, and in black patients, the OS outcomeremained unchangedin the targeted therapy era as compared to the pre-targeted therapy time period.
DISCUSSION
The results of our SEER analysis reveal distinct OS outcomes within the different time periods; 2000–2003 and 2005–2008. In recent years, there has been a substantial change in the management of advanced RCC with the availability of the current armamentarium of targeted agents including VEGF, TKI and mTOR inhibitors. When compared to immunotherapy alone, targeted therapy is universally applicable, feasible and tolerable, but carries cumbersome toxicities and a hefty economic burden. (3–9). However it has created a favorable impact on OS.
The SEER database analysis reported in this paper revealed that the advent of targeted therapies improved OS in certain population subgroups, but widened the disparity gap in others. Specifically, the advanced RCC patients of black race, and those without nephrectomy, did not appear to benefit from targeted therapy. The reasons for the racial disparity are likely to be multifactorial. Other studies have reported racial disparity in clinical outcomes in RCC, even prior to the availability of targeted therapy. We reported on a retrospective review within the clinical trial population of advanced RCC patients and noted that black patients had a shorter OS (median OS = 6.9 months) as compared to the white cases (median OS 11.5 months). 12 month OS rates were 49% and 12% for white and black patients, respectively[10]. Since all patients were enrolled on clinical trials, some other confounders such as performance status and access to care were controlled for, but despite this, the disparity persisted. The differences in natural history of disease could be likely reasons. [10]
Black patients derived minimal improvement in OS, evenin the targeted therapy era. This may possibly be due to lack of access, or inability to sustain or tolerate treatment due to associated comorbidities and toxicities. Genomic differences in disease may also be contributing to the disparities in outcome. A SEER analysis from the pre targeted therapy era, reported that the magnitude of difference in OS was largest between black and white patients younger than 60 years of age, with localized RCC. The median survivals noted in these subgroups were 190 and 259 months, respectively (P 0.0001). Black patients had a greater estimated annual percentage increase in incidence (4.46% for 20 to 59 years and 4.35% for 60 years) compared with white patients with localized RCC (2.87% and 3.06%, respectively) [11]. Young black patients with localized renal cancer had a greater rate of rise in incidence, and a poorer outcome, than white patients within the same subgroup of age and disease stage. This reveals that the racial disparity was already present prior to availability of targeted therapies, and has continued into the targeted therapy era.
Racial disparity in RCC could be accounted for by differences in nephrectomy status, access to targeted therapy, and the presence of multiple comorbidities such as hypertension which could impede administration of VEGF inhibitors. Interventions for each of these need to be planned in future studies, with the objectives of narrowing the survival disparities noted within the advanced RCC population. In the cytokine therapy era, nephrectomy altered outcome in advanced RCC. This was shown by the results of 2 randomized trials that demonstrated significant OS improvement with nephrectomy [12, 13] It follows from the results of our SEER analysis that nephrectomy plays a role in altering OS outcomes even in the targeted therapy era. Other population database trials have also demonstrated the importance of nephrectomy in changing OS outcomes in advanced RCC [14]. In our study, patients who did not undergo nephrectomy, had a greater risk of death in the targeted therapy era, than the pre-targeted era, suggesting that the advanced RCC patients without nephrectomy did not derive a survival benefit from the targeted therapy. Whether this was because they did not receive the therapy, or simply failed to respond to the therapy, is unknown with this analysis. The longer survival outcomes in patients who were able to receive nephrectomy could also be selection bias. Patients with better performance status, good renal reserve, less metastatic burden, and fewer major comorbidities, are likely to be surgical candidates and hence selected for nephrectomy. The same factors would make them more likely to receive and tolerate systemic targeted therapy.
The main purpose of our study was to evaluate the impact of the novel targeted agents on the survival of advanced RCC population, to determine if benefits seen in clinical trials applied to the general population. We found that these agents impart a significant survival benefit. In the advanced RCC cases the median survival improved by 5 months from 15 to 20 months (p<0.001) when comparing the two treatment periods. Similar results were seen in two Canadian clinical trials [15,16]. Heng et al [15] conducted a retrospective analysis to examine the effect of sunitinib (VEGF TKI) in the British Columbia (BC) cancer registry. Of 200 advanced RCC patients identified, 131 patients were treated with interferon, and 69 patients were treated with sunitinib. There was a significant improvement in the median OS of patients treated with sunitinib (17.3 months vs. 8.7 months, p= 0.004). This study confirmed the efficacy of targeted therapy in an unselected population. The other Canadian study [16] reported the survival impact of tyrosine kinase inhibitor therapy (sorafenib and sunitinib) in metastatic RCC population in Alberta, Canada. In this study systemic therapy was associated with improved OS in both first and second line settings [16]. Compared to our study, the two Canadian studies have smaller sample sizes and are limited to specific areas in Canada. However they have specific information regarding the treatment agent. The information regarding specific therapies, histology, prognostic factors is not captured in the SEER database. A third, more recent study was reported from the California registry [17] by Shek et al. and compared OS outcomes between the time periods of 1998–2003 with 2004–2007, labeled by the authors as the “cytokine” and “post-cytokine” eras respectively. Our study corroborates their findings of about a 5% improvement in 3 year OS in the “post-cytokine” era. Besides being confined to the RCC population within California only, the report by Shek et al, was not restricted to advanced RCC cases and only about 30–40% of the population analyzed had advanced RCC. Since targeted therapy is an approved standard only in advanced cases of RCC, the impact of targeted therapy use could not be detected in more than two-thirds of the population studied. In fact, multivariate analysis in the study revealed that localized disease (HR= 18.1), nephrectomy (HR= 2.87), and clear cell histology (HR=1.33), predicted for improved OS. Our SEER study encompasses a nationwide population of advanced RCC alone, within limited time periods, making it more specific towards assessing the impact of targeted therapy on clinical outcomes.
The main strengths of our study are the large sample size, the quality and reliability of the data, and population-based nature of the SEER database, which reflects the clinical outcomes of RCC outside the realm of clinical trials. However, there are also several limitations to our study. Data on the precise systemic agents utilized is not available in SEER; therefore we could not directly evaluate survival differences between those receiving specific targeted therapies. Also, SEER routinely collects only first course of treatment. As subsequent treatment information was not available, progression free survival could not be evaluated. In addition, some of the factors such as calcium, lactate dehydrogenase and hemoglobin levels that are included in the Motzer [18] or Heng [19] prognostic scores, are not collected by SEER and could have an impact on OS. Finally, we have only 3 years of follow-up for OS estimates and longer follow up will provide mature data regarding extent of clinical impact of targeted therapy.
In conclusion, targeted therapy has made a positive impact on clinical outcomes in advanced kidney cancer. There is a consistent OS benefit noted in the 2005–2008 time period (post targeted therapy), as compared to the pre-targeted therapy era. Supportive care has not changed significantly enough to account for this difference. Nephrectomy continues to be a key determinant of OS even in the targeted therapy era. Simultaneously the disparity gap is widening. Blacks with advanced RCC and patients without nephrectomy, are less likely to benefit from targeted therapies. Possible interventions include improving access to care, and better multidisciplinary expertise targeting high risk patient populations. Clinical trial focus on specific patient subgroups with poor prognosis should be prioritized.
Acknowledgments
Supported in part by NCI grant # HHSN261201000028C
Footnotes
Disclosures and Conflicts: No financial disclosures for the authors or funding sources for the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63 :11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 4.McDermott DF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23 :133–41. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356 :125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356 :115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi RT, Heilbrun LK, Jain V, Vaishampayan UN. Racial disparity in outcomes of a clinical trial population with metastatic renal cell carcinoma. Urology. 2006;68 :296–301. doi: 10.1016/j.urology.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Vaishampayan UN, Do H, Hussain M, Schwartz K. Racial disparity in incidence patterns and outcome of kidney cancer. Urology. 2003;62 :1012–7. doi: 10.1016/j.urology.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345 :1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 13.Mickisch GH, Garin A, van Poppel H, et al. European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358 :966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 14.Small AC, Tsao CK, Moshier EL, et al. Trends and variations in utilization of nephron-sparing procedures for stage I kidney cancer in the United States. World J Urol. 2012 May 24; doi: 10.1007/s00345-012-0873-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heng DY, et al. A population-based study evaluating the impact of sunitinib on overall survival in the treatment of patients with metastatic renal cell cancer. Cancer. 2009;115:776–83. doi: 10.1002/cncr.24051. [DOI] [PubMed] [Google Scholar]
- 16.Warren M, et al. A population-based study examining the effect of tyrosine kinase inhibitors on survival in metastatic renal cell carcinoma in Alberta and the role of nephrectomy prior to treatment. Can Urol Assoc J. 2009;3:281–9. doi: 10.5489/cuaj.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shek D, Tomlinson B, Brown M, et al. Epidemiologic Trends in Renal Cell Carcinoma in the Cytokine and Post-Cytokine Eras: A Registry Analysis of 28,252 Patients. Clin GU Cancer. 2012;10 :93–8. doi: 10.1016/j.clgc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27 :5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22 :454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]